At the University of Leeds, researchers have devised a new method that could aid in the development of a new generation of protein-based synthetic biomaterials.
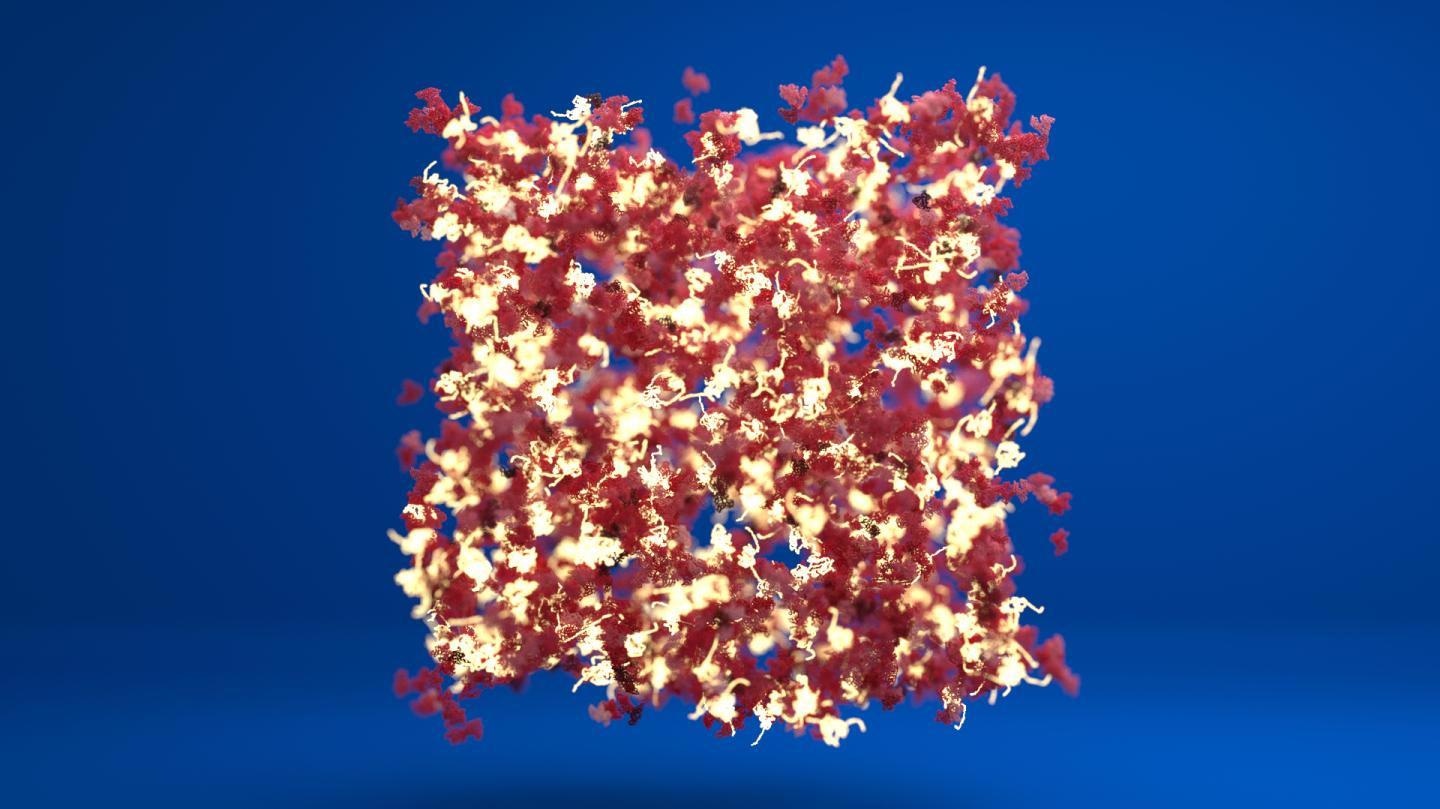 A schematic of a protein hydrogel showing regions of folded protein (in red) connected by regions of unfolded protein (in white). Image Credit: Lorna Dougan/Phospho Animations.
A schematic of a protein hydrogel showing regions of folded protein (in red) connected by regions of unfolded protein (in white). Image Credit: Lorna Dougan/Phospho Animations.
The biomaterials could find use in joint repair, wound healing and other areas of healthcare and food production in the future.
Controlling and fine-tuning the way protein building blocks combine into complex protein networks, which constitute the basis of biomaterials, is one of the most difficult issues.
Leeds researchers are looking at how changes in the structure and mechanics of individual protein building blocks — changes at the nanoscale — might modify the structure and mechanics of the biomaterial at a macro level while preserving the protein network’s biological function.
The researchers state that they were able to change the structure of a protein network by deleting a specific chemical link in the protein building blocks. These bonds were named “protein mainstays” by the researchers. The findings have been described in a paper published in the scientific journal ACS Nano.
When the protein staples were removed, the individual protein molecules were able to interact and organize into a network more easily. This created a network of folded protein regions joined by unfolded protein regions, making the biomaterial exhibit widely diverse mechanical properties.
Proteins display amazing functional properties. We want to understand how we can exploit this diverse biological functionality in materials which use proteins as building blocks. But to do that we need to understand how changes at a nano scale, at the level of individual molecules, alters the structure and behavior of the protein at a macro level.
Lorna Dougan, Professor, School of Physics and Astronomy, University of Leeds
“Controlling the protein building block's ability to unfold by removing the ‘protein staples’ resulted in significantly different network architectures with markedly different mechanical behavior and this demonstrates that unfolding of the protein building block plays a defining role in the architecture of protein networks and the subsequent mechanics,” said Dr. Matt Hughes, lead author of the study, also from the School of Physics and Astronomy.
The researchers employed facilities at Leeds’ Astbury Centre for Structural Molecular Biology and School of Physics and Astronomy, as well as the STFC Rutherford Appleton Laboratory’s ISIS Neutron Muon Source. They were able to spot key changes in the structure of the protein network using neutron beams when the nano-staples were removed.
Dr. Ben Hanson, a Research Associate in the School of Physics and Astronomy at Leeds, modeled the structural changes that were taking place in tandem with the experimental study. He discovered that the act of protein unfolding during network formation was critical in determining the network topology of protein hydrogels.
The ability to change the nanoscale properties of protein building blocks, from a rigid, folded state to a flexible, unfolded state, provides a powerful route to creating functional biomaterials with controllable architecture and mechanics.
Lorna Dougan, Professor, School of Physics and Astronomy, University of Leeds
Journal Reference:
Hughes, M. D. G., et al. (2021) Control of Nanoscale In Situ Protein Unfolding Defines Network Architecture and Mechanics of Protein Hydrogels. ACS Nano. doi.org/10.1021/acsnano.1c00353.