Reviewed by Alex SmithSep 20 2021
NOX (X = 1 or 2) discharged from stationary/mobile sources are often believed as deleterious, anthropogenic originators of ultrafine particulate matters (PM2.5) because NOX can undergo a sequence of SO2-aided photochemical transformative phases to finally become PM2.5, which is an air pollutant.
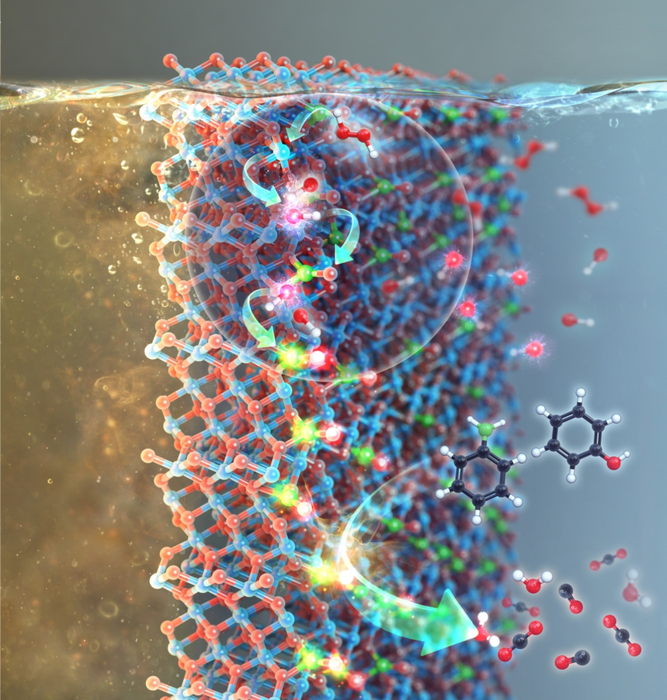 Graphic image of the research. Image Credit: Korea Institute of Science and Technology (KIST).
Graphic image of the research. Image Credit: Korea Institute of Science and Technology (KIST).
A research team from South Korea has recently corrected the general idea of NOX (vide supra) by suggesting an interesting way to make use of NOX in a creative manner.
According to the Korea Institute of Science and Technology (KIST), a research team with principal investigators of Dr. Jongsik Kim and Dr. Heon Phil Ha from the institute has partnered with a research group headed by Prof. Keunhong Jeong in the Korea Military Academy (KMA) to graft NO3− species on a metal oxide through chemical fusion between NOX and O2 under low thermal energy (≤150 °C).
The ensuing supported NO3− species can subsequently be radicalized to produce NO3• analogs that act as degraders of refractory organic substances found in wastewater.
Aqueous recalcitrant compounds that contain bisphenol A and phenolics are usually removed from water matrices through the process of sedimentation using coagulants or through degradation into H2O and COY (Y = 1 or 2) with the addition of OH shuttles such as H2O2, O3, etc.
However, these approaches need more stages to recover coagulants or will have short lifespans and/or chemical volatilities characteristic of •OH, H2O2, and O3, thus drastically hindering the sustainability of H2O purification processes that are being commercialized at present.
As an alternative to •OH, NO3• can be specifically attractive because of its longer lifetime and/or better oxidizing ability than •OH, •OOH, or O2•−, thus being predicted to improve the efficiency in degrading aqueous pollutants over the other radicals mentioned above.
However, NO3• production is not simple and has a few restrictions, such as the need for extremely energized electrons exposed to a radioactive element or highly acidic surroundings.
Dr. Kim and his team have made it feasible under wastewater, including H2O2 and NO3−-functionalized manganese oxide that surface manganese species (Mn2+/Mn3+) primarily stimulate H2O2 for the creation of •OH, whereas •OH then triggers NO3− functionality for its transition into NO3• (represented as •OH → NO3•), all of which are demonstrated by density functional calculation (DFT) methods together with a few control experiments.
The ensuing NO3• species were shown to intensify degradation efficiency of textile wastewater by five- or seven-fold compared to those delivered by conventional radicals (•OH/•OOH/O2•−). Of importance, the catalyst (NO3−-functionalized manganese oxide) discovered here is ~30% less expensive than a conventional commercial catalyst (iron salt) and can be mass-produced.
Most significantly, the catalyst can be reused 10 times or more. This is in contrast to a conventional catalyst that only promises single utilization in decomposing water pollutants via homogeneous H2O2 scission (•OH generation).
The •OH → NO3• technology has been patented and sold to a domestic company (SAMSUNG BLUETECH). Given a plenty of merits imparted by the catalyst modified with NO3− functionalities, we basically expect to install the catalyst in a wastewater treatment unit so soon.
Dr. Jongsik Kim, Study Principal Investigator, Korea Institute of Science and Technology
This study is funded by the National Research Foundation of Korea (NRF) grant offered by the government (Ministry of Science and ICT, Minister Hye-suk Lim) and the Future R & D and Young Fellow grants offered by KIST.
Journal Reference:
Kim, J., et al. (2021) Deciphering Evolution Pathway of Supported NO3 radical Enabled via Radical Transfer from •OH radical to Surface NO3− Functionality for Oxidative Degradation of Aqueous Contaminants. JACS Au. doi.org/10.1021/jacsau.1c00124.