A sensational new solar material known as organic-inorganic halide perovskites could soon enable the United States to realize its solar goals and decarbonize the power grid. Perovskite solar materials are one thousand times thinner than silicon and can be altered to respond to various colors of the solar spectrum just by changing their composition mix.
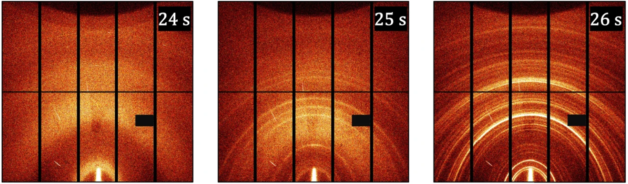 A new technique at the Advanced Light Source reveals what happens (from left to right) in the second before, during, and after a drop of a solidifying agent transforms a liquid precursor solution into a perovskite solar material. Image Credit: Berkeley Lab
A new technique at the Advanced Light Source reveals what happens (from left to right) in the second before, during, and after a drop of a solidifying agent transforms a liquid precursor solution into a perovskite solar material. Image Credit: Berkeley Lab
Usually fabricated from organic molecules such as methylammonium and inorganic metal halides such as lead iodide, hybrid perovskite solar materials have a high tolerance for flaws in their molecular structure and absorb visible light more capably than silicon, the solar sector’s standard.
Overall, these features make perovskites potential active layers not only in photovoltaics (technologies that turn light into electricity), but also in other types of electronic instruments that react to or control light including light-emitting diodes (LEDs), lasers and detectors.
But “although perovskites offer great potential for greatly expanding solar power, they have yet to be commercialized because their reliable synthesis and long-term stability has long challenged scientists,” said Carolin Sutter-Fella, a researcher at the Molecular Foundry, a nanoscience user facility at Lawrence Berkeley National Laboratory (Berkeley Lab). “Now, a path to perfect perovskites may soon be within reach.”
A recent Nature Communications study co-led by Sutter-Fella states that solar materials production could be assisted by an advanced new instrument that uses two kinds of light — visible laser light and invisible X-ray light — to investigate a perovskite material’s crystal structure and optical characteristics as it is synthesized.
When people make solar thin films, they typically have a dedicated synthesis lab and need to go to another lab to characterize it. With our development, you can fully synthesize and characterize a material at the same time, at the same place.
Carolin Sutter-Fella, Scientist, Molecular Foundry, Lawrence Berkeley National Laboratory
For this study, Sutter-Fella drew together an international team of renowned researchers and engineers to equip an X-ray beamline endstation with a laser at Berkeley Lab’s Advanced Light Source (ALS).
The highly intense X-ray light of the new instrument allows scientists to test the crystal structure of the perovskite material and reveal details about the rapid chemical processes. For example, it can be employed to characterize what occurs in the second before and after a drop of a solidifying agent converts a liquid precursor solution into a solid thin film.
Simultaneously, its laser can be employed to develop electrons and holes (electrical charge carriers) in the perovskite thin film, allowing the researchers to view a solar material’s reaction to light, whether as a finished product or during the transitional stages of material synthesis.
Equipping an X-ray beamline endstation with a laser empowers users to probe these complementary properties simultaneously.
Carolin Sutter-Fella, Scientist, Molecular Foundry, Lawrence Berkeley National Laboratory
This combination of concurrent measurements could become part of a programmed workflow to track the making of perovskites and other functional materials in real-time for process and quality control.
Perovskite films are usually made by spin coating, an inexpensive method that does not require costly equipment or complex chemical setups. The case for perovskites becomes even brighter when one considers how energy-intensive it is merely to synthesize silicon into a solar device. While silicon needs a processing temperature of approximately 2,732°F, perovskites can be effortlessly synthesized from solution at ambient temperature to just 302°F.
First author Shambhavi Pratap, who studies the use of X-rays to analyze thin-film solar energy materials, had a critical role to play in creating the instrument as an ALS doctoral fellow. She recently finished her doctoral studies in the Müller-Buschbaum group at the Technical University of Munich.
“The instrument will allow researchers to document how small things that are usually taken for granted can have a big impact on material quality and performance,” Pratap said.
To make reproducible and efficient solar cells at low cost, everything matters.
Carolin Sutter-Fella, Scientist, Molecular Foundry, Lawrence Berkeley National Laboratory
She added that the research was a team effort that traversed a broad range of scientific disciplines.
The study is the most recent chapter in a body of work for which Sutter-Fella received the Berkeley Lab Early Career Laboratory Directed Research and Development (LDRD) Award in 2017.
We know that the research community is interested in using this new capability at the ALS. Now we want to make it user-friendly so that more people can take advantage of this endstation.
Carolin Sutter-Fella, Scientist, Molecular Foundry, Lawrence Berkeley National Laboratory
The device design was headed by ALS senior scientific engineering associate Jonathan Slack. Peter Müller-Buschbaum of the Technical University of Munich co-led the research. Other co-authors include Tze-Bin Song, Finn Babbe, Camelia Stan, Zhenghao Yuan, Tina Long, Nicola Barchi, Zach Haber and Nobumichi Tamura.
This study received support from the DOE Office of Science, Berkeley Lab’s Early Career Laboratory Directed Research and Development program, the German Research Foundation and the Bavarian Collaborative Research Project Solar Technologies Go Hybrid (SolTech) program.
The Advanced Light Source and Molecular Foundry are DOE National User Facilities at Berkeley Lab.
Using X-ray Light to Study Solar Materials at the Advanced Light Source
In this 2019 video, first author Shambhavi Pratap of the Technical University of Munich discusses how she studies thin-film solar energy materials using X-rays at the ALS. Video Credit: Marilyn Sargent/Berkeley Lab
Journal Reference:
Pratap, S., et al. (2021). Out-of-equilibrium processes in crystallization of organic-inorganic perovskites during spin coating. Nature Communications. doi.org/.10.1038/s41467-021-25898-5.