A research group, led by Prof. Jeff Dahn of Dalhousie University, is focused on a thorough assessment of the life cycle and properties of pouch cells after the incorporation of graphite. The research was published in the Journal of the Electrochemical Society.

Study: Impact of Graphite Materials on the Lifetime of NMC811/Graphite Pouch Cells: Part I. Material Properties, ARC Safety Tests, Gas Generation, and Room Temperature Cycling. Image Credit: Miriam Doerr Martin Frommherz/Shutterstock.com
According to the novel study, strategic research regarding lifecycle enhancement of storage devices and cells has not been made, as it is quite expensive requiring substantial resources.
Recent Studies
Often, researchers are interested in Li-ion cells with enhanced high-energy-density characteristics. Most recently, the focus has been on Ni-rich cells, whose density characteristics have been enhanced by the inclusion of additive doping materials or via the process of cathode coatings.
Limitations of Ni-Rich Materials
Despite the interest of researchers worldwide, nickel-rich materials show rapid lattice volume volumetric variations during the charging and discharging processes that are the core source of particle microcracking at the molecular level and recurring drainage reactions with the electrolytes leading to cell failure.
Electrolyte additives have been incorporated for inhibiting lithium loss, but graphite has been the center of focus to diminish the parasitic reactions that cause a significant reduction in the lifecycle.
Importance of Graphite
Graphite is a mineral that is found in igneous rocks. The most common uses of graphite include in battery, metallurgy, expanded graphite, brake linings, casting, and lubricant applications. Graphene, a naturally occurring component of graphite, has unusual physical qualities and is one of the toughest compounds known.
Types of Graphite Material
Natural Graphite (NG) and artificial or synthetic graphite (AG) are the two major types of graphite. Natural graphite is mostly obtained from the Earth’s crust via mining techniques, whereas a carbon precursor and petroleum coke treatment is used for the synthesis of artificial graphite. Artificial graphite has a slightly lower specific heat capacity than naturally occurring graphite.
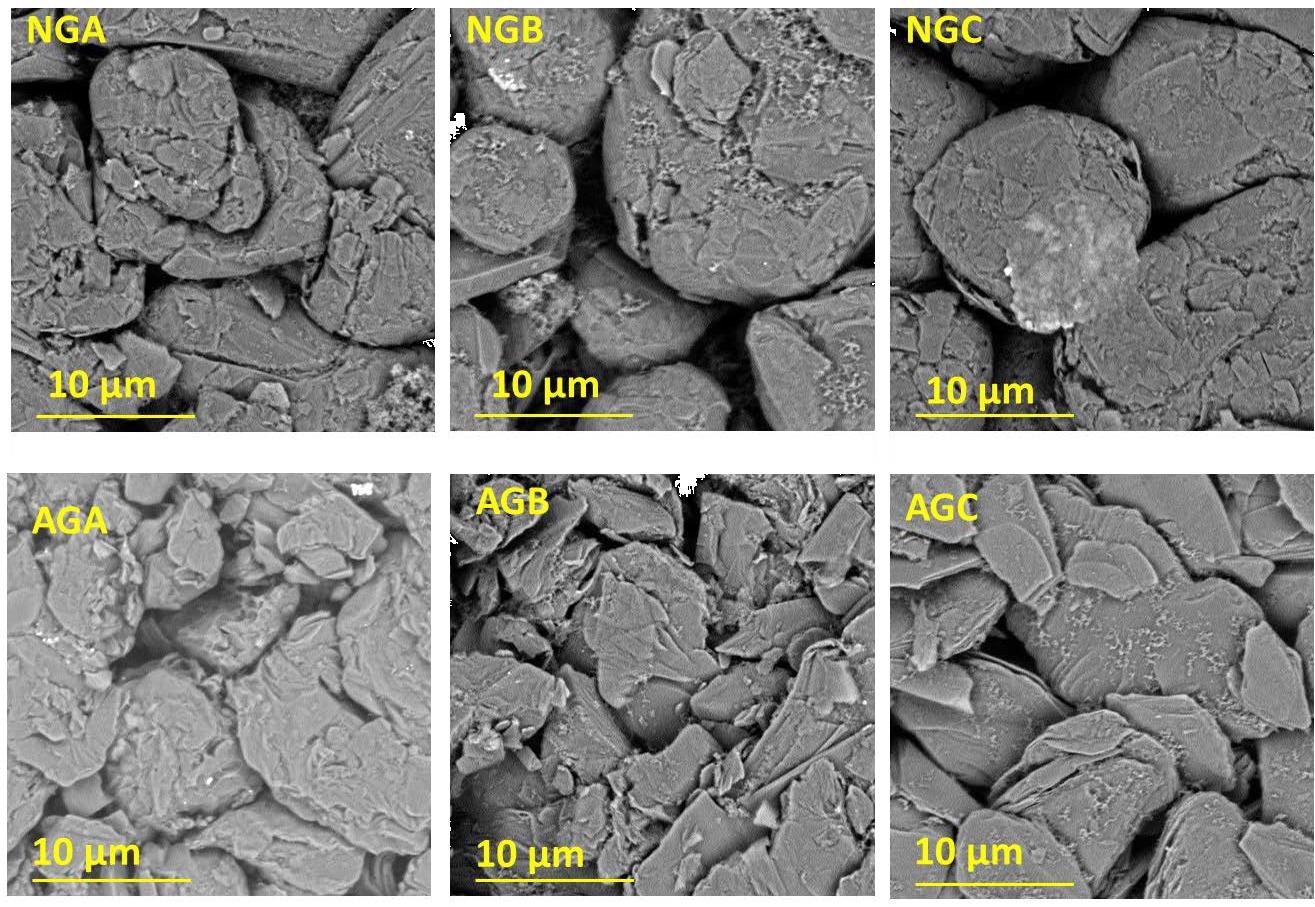
SEM images of the different 169 graphite electrodes in this work. Image Credit: Eldesoky, A., Journal of the Electrochemical Society
The graphite electrodes in a cell or a battery significantly affect its charging and discharging rate as compared to other materials, its SEI composition, lithium loss, gas formation, and lifecycle of the battery.
The recent research published by the researchers was conducted via a complete analysis of the impact of three natural graphite and two artificial graphite on NMC811 pouch cells.
Introduction to Pouch Cells
The pouch cell is a pyramidal device with an aluminum polymer film membrane on the outer coating of liquid or semi-solid lithium-ion; it does not have a metallic protective case. As a result, as opposed to regularly available square cells, the weight may be reduced, and it can be readily manufactured in a variety of sizes and configurations.
Laminate pouch cells are extensively employed in retail electronic goods (e.g., smartphones), where the longevity requirements are significantly lower than in the automobile industry.
Research Findings
The researchers utilized comprehensive techniques for analysis. Machine-made pouch batteries with a rated capacity of 240 mAh and no electrolyte were used.
It was observed that the natural graphite material had a round molecular topology while AG had flake-like particle morphology and had a more tightly dense packing. NG showed a higher degree of crystallinity with only 3-4% of misalignment probability.
AGs appear to have had no carbon coating applied to them, but NGs appear to have a large coating. The coating put on NGs accounts for 5% of the total weight. It was also demonstrated that carbon-coated NG performed better than uncoated materials.
The experiments showed that graphite types with low reactivity can impede electrolyte reduction. As a result, a thoroughly passivated graphite with a strong SEI will limit the rate of parasitic processes in the cell. AGC had very little reduction activity, indicating that it has a very tiny contact area.
CO2 and C2H4 gases were discovered as the dominating gases in all cells, coupled with Ar since the cells were supplied with electrolytes in an Ar glovebox.
The cells demonstrated good RT efficiency, which is to be expected for cells made with cutting-edge ingredients and outstanding additive mixes; nonetheless, certain discrepancies amongst graphite compounds can still be noticed.
Limitations
Although the research showed advantageous results, certain limitations still exist regarding the processes incorporated. Because these units were evaluated at room temperature with no temperature regulation, noisy data from temperature variations increases uncertainty. These certainties include overvaluation of lifetime and capacities and proper steps should be taken to incorporate these discrepancies. Also, data from coin cells and pouch cells revealed that the AGC and NGB had extremely limited electrolyte reduction activity.
In short, graphite is advantageous for pouch cells, which is thoroughly shown by the novel research.
References
Eldesoky, A., Bauer, M., Azam, S., Song, W., Weber, R., Hy, S., . . . Dahn, J. R. (2021). Impact of Graphite Materials on the Lifetime of NMC811/Graphite Pouch Cells: Part I. Material Properties, ARC Safety Tests, Gas Generation, and Room Temperature Cycling. Journal of the Electrochemical society. https://iopscience.iop.org/article/10.1149/1945-7111/ac39fc/meta
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.