Even though the viability of some biochars for pollutant adsorption isolation has been demonstrated, according to the most recent study published in the journal Materials, not all possible biomasses have been studied and described.

Study: Physicochemical Characterization of Cherry Pits-Derived Biochar. Image Credit: c12/Shutterstock.com
Effect of Hazardous Biomasses
Aquatic organometallic toxicants provide several risks to human civilization, including pulmonary allergens, skin irritations, digestive difficulties, sterility, and malignancy. Organic pollutants can be metabolized, destroyed, or combusted, but artificial contaminants like toxic substances are redistributed throughout the ecosystem.
Although toxic heavy metals exist naturally in the atmosphere, their quantities are now often enhanced as a result of increased industrialization. Toxic materials infiltrate the soil nutrients via water sources, where they then infect the food supply and, eventually, humans. Because wastes and wastewater are the primary sources of heavy metals, they must be treated to encapsulate contaminants and/or transform them into less hazardous forms.
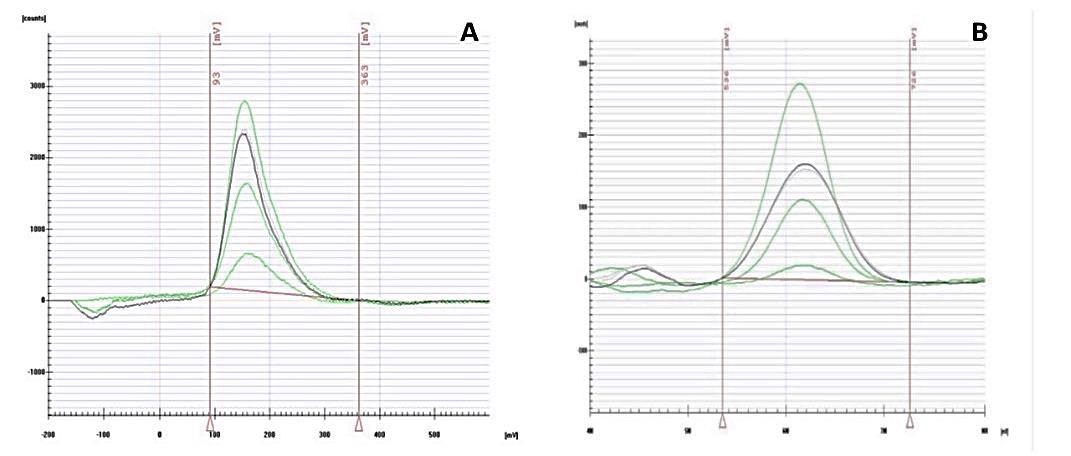
Characteristic chronopotentiogram for the determination of As (A) and Hg (B) in supernatant samples after sorption by CP, CPB, and AC-based adsorbents. Image Credit: Frišták et. al., Materials
What is Activated Carbon (AC)?
Owing to its useful qualities and characteristics, widely accessible activated carbon as a sorption material has piqued the interest of many in the field of treating wastewater and filtration. Activated carbon, referred to as the activated form of charcoal, is a type of carbon that has been treated to have microscopic, reduced holes that enhance the accessible surface area for sorption or redox composition.
Thermal properties, homogeneous surface characteristics, minimal toxicological risk, pore-volume, and thus the high surface area of activated charcoal all influence its numerous industrial applications procedures. However, the cost of manufacturing activated carbon is rather expensive, owing primarily to post-production activation operations using physicochemical means.
Introduction to Biochar
Biochar is a coal-like compound produced from the combustion of organic waste from agriculture and forestry (also known as biomass) in the lack of oxygen. Biochar, the primary byproduct of thermal conversion procedures, is a carbon- and nutritionally abundant substance that is appropriate for farming techniques, as well as being particularly safe for humans, wildlife, and vegetation.
Because of the enhanced porous surface area caused by the thermal transformation, it may be used as a soil fertilizer, immobilization reagent, and possible novel adsorption substance for the immediate removal of various pollutants from the polluted aqueous phase and industrial effluents.
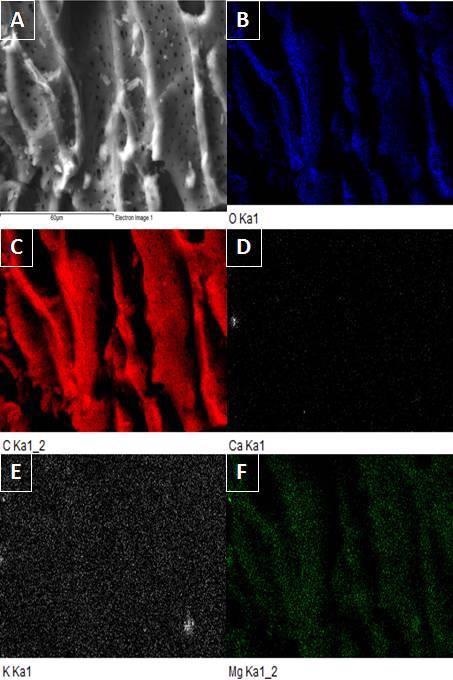
SEM image of CPB (A) and EDX mapping of selected area of CPB for O (B), C (C), Ca (D), K (E), and Mg (F). Image Credit: Frišták et. al., Materials
There has been a significant scientific advancement in the region of biochar utilization in the past few decades. The primary center of attention has been on processing parameters improvements, such as heating rate, residence duration, and material provenance.
Utilization of Biomass for Biochar Production
A wide range of biomass feedstock may be utilized to create biochar under stringent anaerobic conditions at combustion temperatures ranging from 350 to 700 degrees Celsius. Agricultural debris, fiber vegetation scrap from fresh cellulose manufacturing, industrial wastes, organic wastes, plant leftovers, and decaying matter or their components are all viable feedstocks.
In general, the conversion of waste biomass into biochar and AC creates a revenue source from otherwise wasted waste, increases fertilizer resource utilization and soil resilience, reduces drinking water contamination, and reduces the environmental implications of industrialized agricultural production.
This biomass transformation would employ waste from domestic sources to moderate domestic problems, decreasing the need for long-distance transportation of wastes and byproducts while improving human health and the ecological systems.
The Utilization of Cherry Pit Biomass (CPB)
In recent years, the maximum fresh cherry output in the EU has been around 794,000 metric tons per year. Because of the concentration of saturated fats, cherry pit (CP) oils are encouraged to be used in skincare and cookery. Researchers have discovered the possibility of decomposition of cherry pit waste for recuperation as alternative energy, as well as the possibility of co-firing biomass with coal to create power.
Cherry pit bio-waste has also been used as catalytic material for electrochemical applications, alkali gaseous biosorbents, and soil supplements for greenhouses crops. Sadly, despite many advantages, there have been few investigations on the extraction of cherry pits for use as activated carbon.
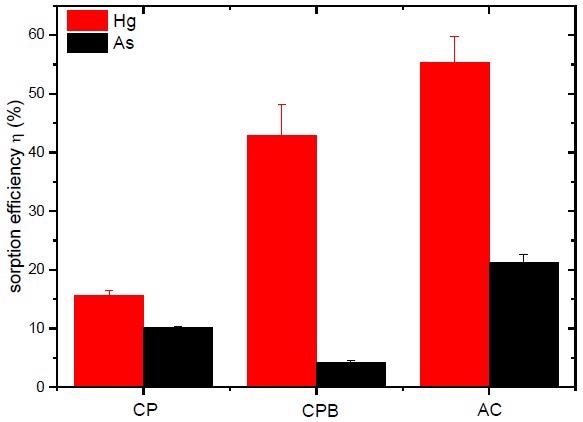
Efficiency of Hg and As sorption separation from aqueous solutions by CP, CPB, and AC under the following experimental conditions: sorbent to sorbate ratio 1:40, sorbent fraction 250–1000 µm, c0 (Hg) = 0.5 mmol/L, c0 (As) = 0.2 mmol/L; pH0 = 5.5–6.0; contact time 24 h at 22 ± 2°C, stirring 45 rpm. Image Credit: Frišták et. al., Materials
Given this circumstance and the need for more knowledge to inform future research and industry practices, the most recent work concentrated on the synthesis and characterization of cherry pit-derived biochar as a precursor for activated carbon via chemical or physical activation.
Research Findings
The sample analysis revealed that there was an increase in overall carbon in CPB when contrasted to CP. There was a substantial change in the porous nature of the pyrolysis sample, as well as the formation of novel surface morphology. When contrasted to the other samples, the CPB sample had the largest amount of phenolic chemicals.
It has been reported that the temperature of pyrolysis has a considerable influence on biochar sorption. Biochar manufactured at 600 degrees Celsius exhibited a lower adsorption capacity than biochar made at 300 degrees Celsius. Furthermore, at lower pH levels, biochar is positively charged, with a different extent of protonation of organic compounds than at higher pH levels.
In summary, slow pyrolysis at 500 C was used to develop a combustion byproduct that was investigated as a potentially helpful substance in adsorption separations of pollutants from water. However, the results imply that biochar-based materials generated from cherry pits might be used in cationic pollutant sorption separations. The adsorption of anionic chemical components of sorbates might be enhanced by physically or chemically modifying the surface of biochar.
Further Reading
Frišták et. al. Physicochemical Characterization of Cherry Pits-Derived Biochar. Materials 2022. 15(2). 408. Available at: https://www.mdpi.com/1996-1944/15/2/408
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.