The common household antiseptic, hydrogen peroxide, utilized to clean scratches and cuts, can also influence space shuttles.
 To produce safer, more economic, and environmental hydrogen peroxide, an international research team turned to copper. Image Credit: Nano Research.
To produce safer, more economic, and environmental hydrogen peroxide, an international research team turned to copper. Image Credit: Nano Research.
Although the version being sold in pharmacies is far less concentrated when compared to the one used in the industry, the simple reduction of two oxygen and two hydrogen atoms into the water and extra oxygen could help generate huge results.
The compound’s production seems to be expensive, though, needing highly-priced metals to activate the essential chemical reactions that, when left free, could produce accidental explosions.
To produce safe, highly affordable, and environmental hydrogen peroxide, an international research group turned its focus to copper. The common metal helped decrease the number of manufacturing steps, thereby making the consecutive hydrogen peroxide highly stable, profitable and efficient. The study was published on January 11th, 2022, in the Nano Research journal.
According to Qian Liu, a study author and associate professor at Chengdu University’s Institute for Advanced Study, hydrogen peroxide is a high-value oxidant — a substance that exhibits the potential to accept electrons from other substances. It is conventionally produced via a multi-step process in which an expensive metal, like palladium, electrochemically responds with a chemical compound consisting of oxygen and hydrogen to decrease the oxygen’s electrons by four to generate hydrogen peroxide and undesirable organic waste.
The four-electron reduction process generates hydrogen peroxide and water — there is competition between the two processes. As such, in the process of designing and preparing catalysts, we need to satisfy a two-electron reaction process to selectively produce hydrogen peroxide as much as possible to reduce unnecessary energy loss.
Qian Liu, Study Author and Associate Professor, Institute for Advanced Study, Chengdu University
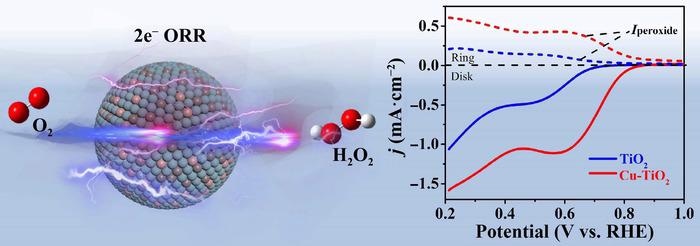
The scientists utilized titanium dioxide as an abundant, non-toxic and stable catalyst, but had to advance it to attain a two-electron reaction process.
We doped the titanium dioxide with copper to naturally increase oxygen vacancy concentration, leading to improved electronic conductivity and better generation of hydrogen peroxide.
Shihai Yan, Study Author and Associate Professor, College of Chemistry and Pharmaceutical Sciences, Qingdao Agricultural University
Copper acts as a heteroatom, enabling scientists to control the electronic structure of titanium dioxide. Furthermore, this improved catalyst can make new atomic vacancies in the reduction compounds, thereby promoting one product over another.
For instance, when electrochemically reducing molecular hydrogen and oxygen, the accumulation of copper helps make more spots for oxygen to link with hydrogen to produce hydrogen peroxide. Rather than a competition for the constituents to turn out to be water or hydrogen peroxide, the latter gets a lift, while the rest burns off as gas. When the process is contained in liquid, it is considered a safe side effect.
“Two-electron electro reduction of oxygen into hydrogen peroxide in an aqueous environment provides a safe, sustainable and energy-saving method for on-demand production,” stated Xuping Sun, Professor, Institute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China.
“Copper-doped titanium dioxide exhibits a significantly improved selectivity of up to 91.2% for hydrogen peroxide, meaning that most of components reduce into the desired product. Moreover, it also shows a larger yield and good stability,” Sun added.
Further, the scientists plan to design and synthesize copper-doped titanium dioxide catalysts against practical needs to obtain extensive industrial production.
This study provides a new route to adjust the electronic structure of metal oxide by heteroatom doping as high efficiency electro catalysts for oxygen reduction reaction to produce hydrogen peroxide.
Qian Liu, Study Author and Associate Professor, Institute for Advanced Study, Chengdu University
The other contributors include co-first author Zhiqin Deng, co-first author Li Li, Yuchun Ren, Je Liang, Kai Dong and Tingshuai Li, Institute of Fundamental and Frontier Sciences, University of Electronic Science and Technology of China; Chaoqun Ma, College of Chemistry and Pharmaceutical Sciences, Qingdao Agricultural University.
Additional authors include Yonglan Luo, Institute for Advanced Study, Chengdu University; Bo Tang, College of Chemistry, Chemical Engineering and Materials Science, Shandong Normal University; Yang Liu and Shuyan Gao, School of Materials Science and Engineering, Henan Normal University; and Abdullah M. Asin, Chemistry Department, Faculty of Science & Center of Excellence for Advanced Materials Research, King Abdulaziz University.
This work was financially supported by the National Natural Science Foundation of China.
Journal Reference:
Deng, Z., et al. (2022) Highly efficient two-electron electroreduction of oxygen into hydrogen peroxide over Cu-doped TiO2. Nano Research. doi.org/10.1007/s12274-021-3995-6.