Researchers from Singapore have investigated the catalytic decomposition of methane, a novel technology with can produced hydrogen without emitting carbon dioxide. Published in the journal Energies, they have evaluated the reaction mechanisms of nickel, copper, and iron catalysts, three of the most common types of metal catalysts currently in use.

Study: Decarbonizing Natural Gas: A Review of Catalytic Decomposition and Carbon Formation Mechanisms. Image Credit: Vova Shevchuk/Shutterstock.com
Hydrogen: An Alternative, Green Energy Source
Despite the well-publicized effects of climate change, the world is still reliant on fossil fuels. This reliance brings with it problems with environmental damage and rising global temperatures. In line with international agreements, several countries have developed plans to achieve net-zero by 2050.
Mitigation strategies must meet several demands, including affordability and energy security, as well as reducing damage to the environment and limiting global temperature rises. Several nations are dependent on energy imports to satisfy their domestic and industrial demands, making them particularly vulnerable to energy security issues.
Hydrogen is a clean and versatile energy source and has gained prominence in energy research to meet the demands of a decarbonizing world and avoid future energy gaps. Hydrogen is produced from water using electrolysis. There are several sources of hydrogen gas, and depending on the source, hydrogen gas is classified differently. Green hydrogen comes from renewable sources, grey hydrogen is produced from fossil fuels, and blue hydrogen is produced from a combination of grey sources and carbon capture technologies.

Three stages of hydrogen production. Image Credit: Tong, S et al., Energies
Grey hydrogen is the most commonly produced form, and as it is produced from fossil fuels (typically, natural gas) it is not considered to be completely green, as its manufacture still produces carbon dioxide. However, it is extremely cost-effective, and the technology possesses high conversion efficiency, which has driven the technology’s uptake.
Blue hydrogen is a controversial technology, which is regarded as not widely viable or environmentally benign. However, it can be considered to be a bridging technology between current grey hydrogen production processes and future green hydrogen manufacturing. Green hydrogen provides the possibility of zero carbon emissions by using technologies such as photocatalytic water splitting.
Another hydrogen manufacturing technology that has been researched in recent years is termed turquoise hydrogen. This method uses renewable energy and catalysts to decompose methane into hydrogen and solid carbon mass. Turquoise hydrogen production is another bridging technology that can help to realize true green hydrogen production.
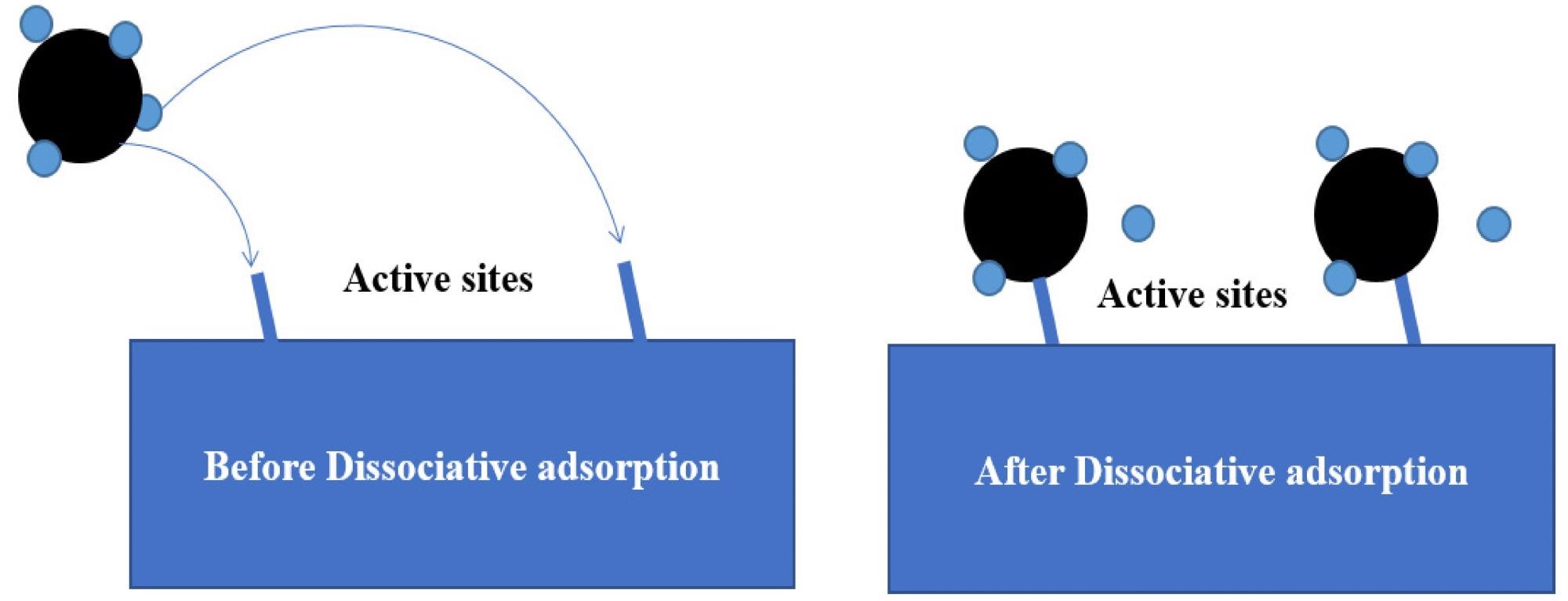
Schematic diagram of the methane dissociative adsorption mechanism model. Image Credit: Tong, S et al., Energies
Catalytic Decomposition of Methane
Catalytic decomposition of methane (CDM) differs from conventional methods of hydrogen production. Steam methane reforming is the most common method used for generating hydrogen. CDM works by breaking carbon and hydrogen bonds to produce hydrogen and solid carbon by-products.
In the absence of catalysts, methane will degrade at temperatures in excess of 1200oC. Catalysts, typically iron, nickel, and cobalt, are used to reduce this reaction temperature, which gradually degrade as the pores in the catalyst bed are filled with solid carbon. To improve the process, a regeneration unit is used to restore catalytic activity. By including a regeneration unit, hydrogen can be continuously produced.
There are some drawbacks to common regeneration strategies such as steam oxidation and air combustion. These methods remove carbon by converting it into carbon dioxide or carbon monoxide, which significantly impacts the green nature of the process.
The Study
The authors have reviewed current research on CDM processes, and the most common catalysts used for producing hydrogen. In the research, the critical aspects of the process have been thoroughly investigated. Detailed descriptions of the methane cracking process (a vital part of CDM) and its reaction mechanism have been provided in the review.
Several studies on the carbon resistance of catalysts and current carbon formation mechanisms have been introduced and discussed in depth in the review. The stated purpose of the study is to provide pertinent information that can guide the development of more effective CDM processes, improve the carbon-metal catalyst ratio, add high value-added carbon products, and provide a route toward improving the reusability of catalysts. The review summarized current methods for regenerating catalysts and discusses potential optimization strategies.
The authors have discussed methane dehydrogenation mechanisms, with dissociative adsorption and non-dissociative absorption investigated and explained. Carbon formation mechanisms covered are the tip growth mechanism, the most common type of carbon deposition, and the base growth mechanism. Additionally, CDM processes can produce special structures such as bamboo-shaped carbon nanotubes and octopus-shaped carbon nanotubes. Formation mechanisms and influencing factors are discussed in the research.
The authors have stated that there are two main challenges that hinder CDM development: the cost of producing hydrogen, and the unstable activity of catalysts. Currently, regeneration methods are immature, so researchers aim to improve CDM’s economic benefits by optimizing the yield and structure of carbon depositions. Alloying elements such as copper can be included to improve the catalyst’s lifetime. Research is ongoing into improving regeneration methods and balancing environmental impact and cost.
In conclusion, the authors have stated that CDM could be a potential bridging technology to achieve hydrogen technology if it can overcome current challenges. Optimizing the processes to achieve renewable hydrogen generation is key to the technology’s maturation.
Further Reading
Tong, S et al. (2022) Decarbonizing Natural Gas: A Review of Catalytic Decomposition and Carbon Formation Mechanisms [online] Energies 15(7) 2573 | mdpi.com. Available at: https://www.mdpi.com/1996-1073/15/7/2573
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.