Reviewed by Alex SmithApr 29 2022
The sustainable artificial upcycling of carbon dioxide (CO2) into value-added products represents an opportunity to address environmental issues and realize a circular economy.
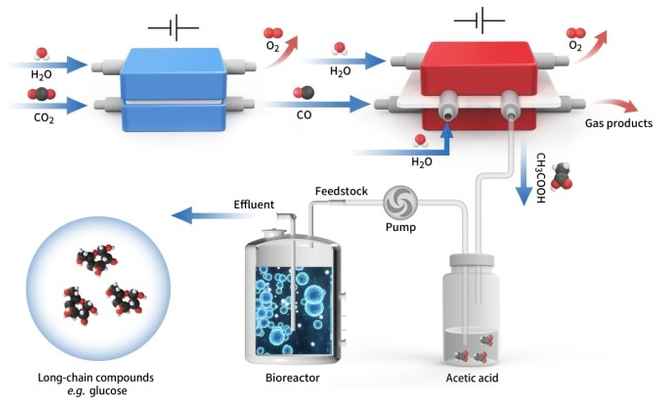 Schematic diagram of in vitro carbon dioxide synthesis of high energy long chain food molecules. Image Credit: Shenzhen Institute of Advanced Technology.
Schematic diagram of in vitro carbon dioxide synthesis of high energy long chain food molecules. Image Credit: Shenzhen Institute of Advanced Technology.
However, in comparison to readily available C1/C2 products, the effectual and long-term synthesis of energy-rich long-chain compounds from CO2 remains a significant challenge.
Professor Chuan Xia of the University of Electronic Science and Technology, Professor Tao Yu of the Chinese Academy of Sciences’ Shenzhen Institute of Advanced Technology, and Professor Jie Zeng of the University of Science and Technology have developed a hybrid electro-biosystem that couples spatially distinct CO2 electrolysis with yeast fermentation to efficiently convert CO2 to glucose.
The observations were published on April 28th, 2022, in the Nature Catalysis journal.
CO2 electrolysis and yeast fermentation are included in the proposed spatially decoupled electro-biosystem. It has a high titer and yield for converting CO2 to glucose or fatty acids.
Acetic acid is not only the main component of vinegar, but also one of the excellent biosynthetic carbon sources. It can be transformed into other substances in life, such as glucose. Acetic acid can be obtained by direct electrolysis of CO2, but with ultra-low efficiency. We thus propose a two-step strategy to convert CO2 into acetic acid, with CO as the intermediate.
Jie Zeng, Professor, University of Science and Technology
As a result, the researchers used a Ni–N–C single-atom catalyst to convert CO2 into CO in a membrane electrode assembly and then produced a grain-boundary-rich Cu (GB_Cu) catalyst for acetate production from electrochemical CO reduction.
In a typical three-electrode flow cell reactor utilizing 1.0 M KOH aqueous electrolyte, GB_Cu showed a high acetate Faradaic efficiency of up to 52% at –0.67 V compared to a reversible hydrogen electrode.
However, the acetate produced by conventional electrocatalytic devices is always mixed with electrolyte salts which cannot be directly used for biological fermentation.
Chuan Xia, Professor, University of Electronic Science and Technology
The investigators devised a porous solid electrolyte reactor with thick anion exchange membranes for pure acetic acid solution purification and separation to meet this challenge. It worked continuously and steadily for 140 hours at a current density of –250 mAcm-2, producing an ultrapure acetic acid solution with a relative purity of ~97% wt.%.
The scientists removed all defined hexokinase genes (hxk1, hxk2, glk1, YLR446W, and emi2) in Saccharomyces cerevisiae in the following microbial fermentation to facilitate microbe growth on pure acetic acid and effectual glucose release in vitro.
The glucose titer was further improved by the overexpression of heterologous glucose-1-phosphatase. With titrated acetate from electrolysis, S. cerevisiae produced an average glucose titer of 1.81 ± 0.14 gL-1, equivalent to a high yield of 8.9 μmol per gram of yeast per hour. S. cerevisiae fed pure acetic acid produced similar results.
Furthermore, titrating acetate from electrolysis was used to feed an engineered S. cerevisiae for the production of free fatty acids, with a total free fatty acids (C8~C18) titer of 500 mgL-1.
The source of carbon for S. cerevisiae fermentation was pure and concentrated acetic acid produced by electrochemical CO2 reduction. Such a platform for long-chain products has the potential to be used on a large scale.
This demonstration is a starting point for realizing light-reaction-free artificial synthesis of important organic products from CO2.
Tao Yu, Professor, Shenzhen Institute of Advanced Technology, Chinese Academy of Sciences
Journal Reference:
Zheng, T., et al. (2022) Upcycling CO2 into energy-rich long-chain compounds via electrochemical and metabolic engineering. Nature Catalysis. doi.org/10.1038/s41929-022-00775-6.