 By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.May 23 2022
By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.May 23 2022In an article recently published in the journal Bioactive Materials, researchers discussed the utility of nano-calcium silicate mineralized fish scale scaffolds for enhancing tendon-bone healing.

Study: Nano-calcium silicate mineralized fish scale scaffolds for enhancing tendon-bone healing. Image Credit: Imageman/Shutterstock.com
Background
Natural tendons/ligaments, which are attached to the bone by unmineralized/mineralized fibrocartilage layers and transmit stresses between bones and between muscle and bone, are known for their excellent toughness and tensile strength.
Tendon grafts/patches have been recommended as a successful technique for aiding the healing of torn tendons and rebuilt tendon-bone interfaces, as well as restoring patients' motor function, for several decades. Current tendon implantation materials, on the other hand, are subpar: most tendon substitutes fail to provide both adequate bioactivity and strong mechanical support.
For the therapeutic treatment of tendon damage, innovatively designed tendon replacements with good bioactivity, biocompatibility, and mechanical qualities capable of speeding native tendon-bone interface regeneration are urgently needed. Surprisingly, several animals have evolved tissues or organs with unique hierarchical microstructures after millennia of development. The most common example is the fish scale (FS).
The synergistic action of deformation mechanisms in the microstructure of FS offers exceptional mechanical characteristics. Natural FS's bioactivity, on the other hand, may be limited because its microstructure has stayed stable in a liquid environment for a long time.
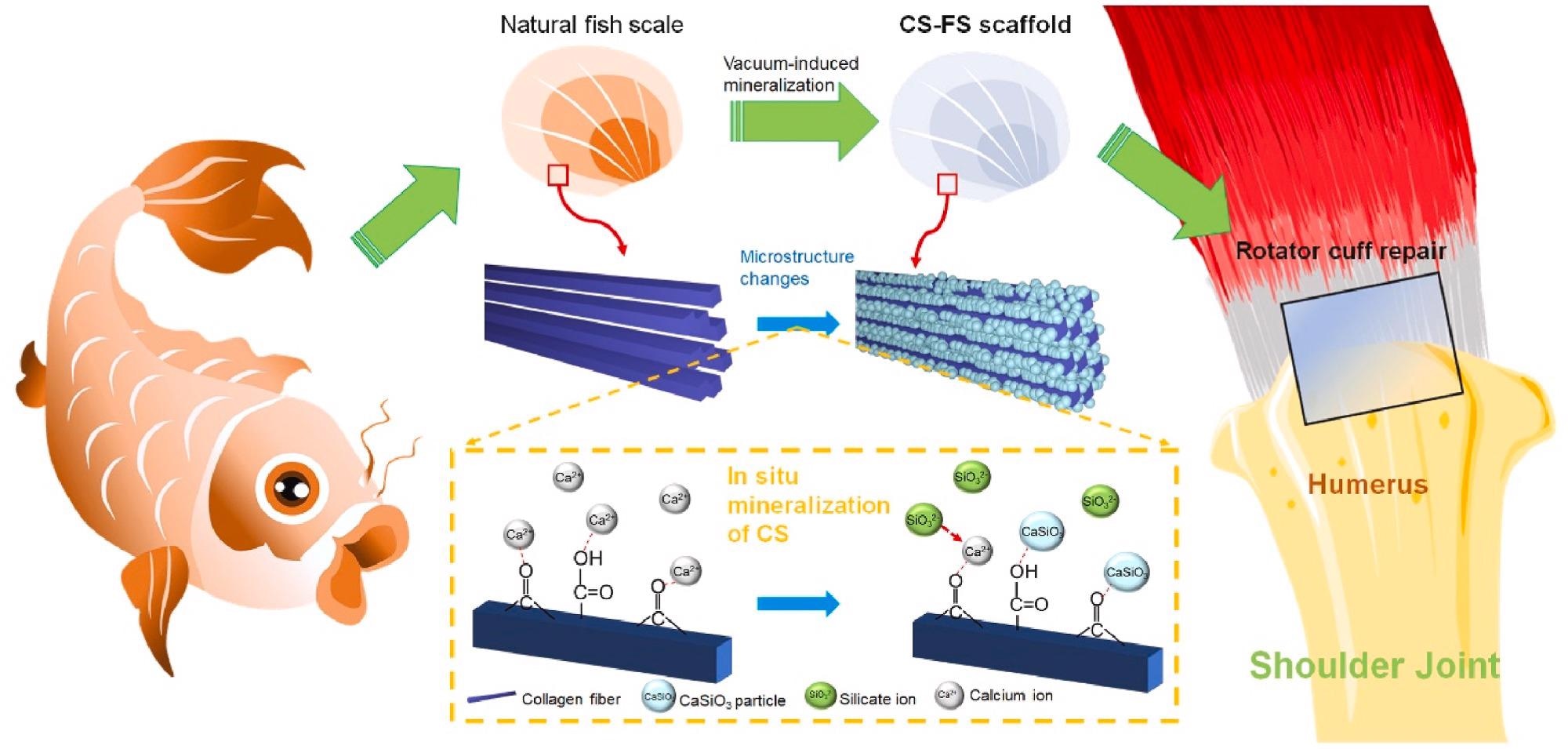
Schematic summarizing work on the fabrication of calcium silicate (CS)-"bioactivated” fish scale scaffold (CS-FS) by vacuum-induced mineralization, for tendon defect repair. In the vacuum-induced mineralization process, Ca2+ ions can engage in coordinating chelation interactions with the carbonyl and carboxyl groups of collagen fibers in fish scale and subsequently combined with SiO32− ions to induce the in situ formation of CS particle. The silicon and calcium ions released from CS-FS contributed to the regeneration of tendon-bone interface and the healing of tendon defect. Image Credit: Han, F et al., Bioactive Materials
About the Study
In this study, the authors discussed the utility of FS modified with calcium silicate nanoparticles (CS NPs) as a new biomaterial (CS-FS), which was inspired by the high-performance exoskeleton of natural creatures. The microstructure and mechanical properties of CS-FS were examined to determine its mechanical qualities.
The researchers performed in vitro investigations to show the ability of silicon and calcium ions to accelerate bone, tendon, and tendon-bone contact regeneration, as well as to demonstrate the bioactivity of CS-FS scaffolds. In both rabbit and rat rotator cuff tear (RCT) models, the therapeutic effects of the CS-FS scaffolds in mending tendon injuries and encouraging tendon-bone interface healing were demonstrated, and the biomechanical properties of the regenerated tissue were assessed.
The team presented a unique bioactivation strategy for the preparation of a calcium silicate-bioactivated fish scale scaffold using a vacuum-induced biomineralization method to evenly mineralize CS NPs on collagen fibers in situ.
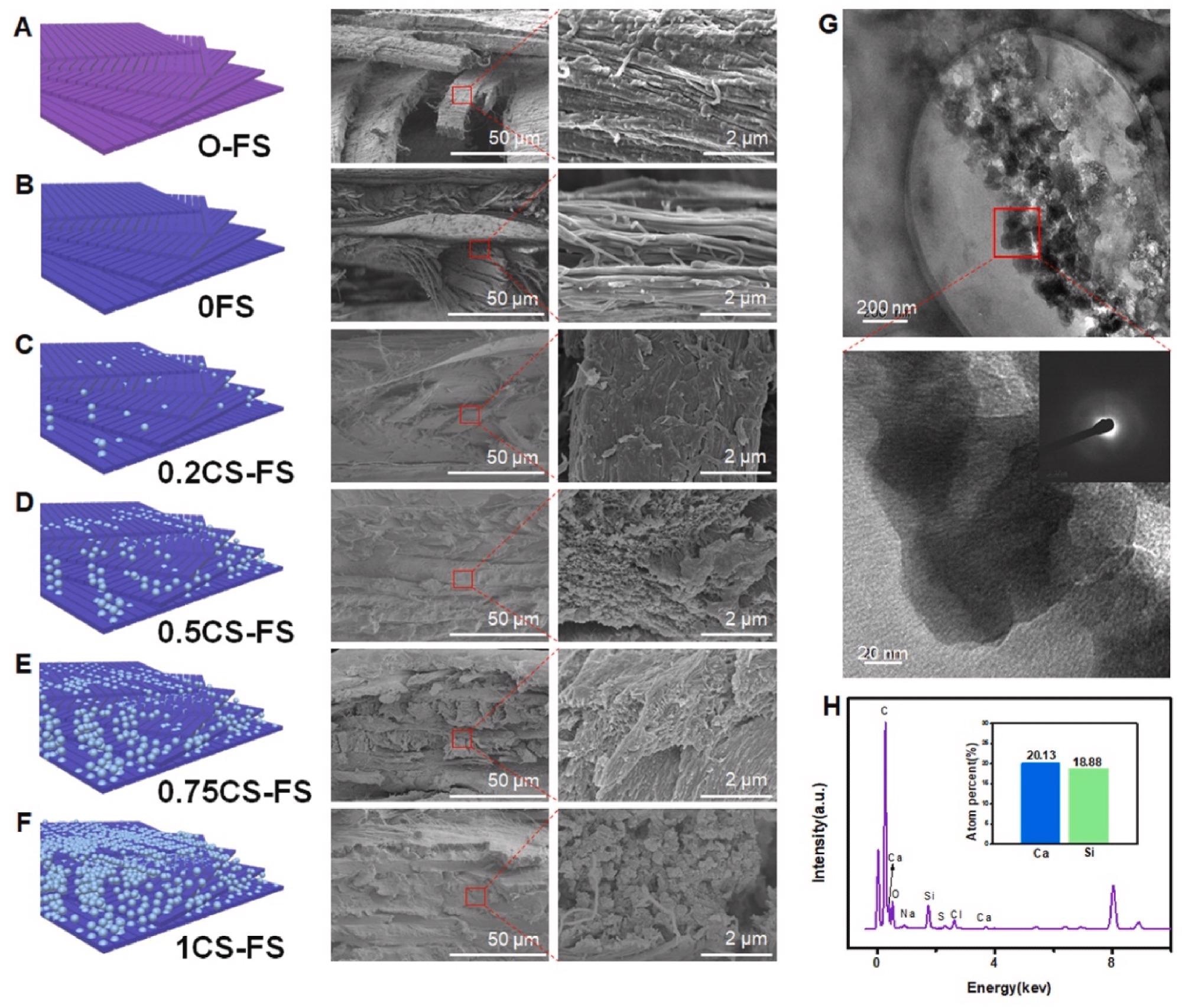
Microstructure and morphology of CS-FS scaffolds with different CS mineralization level. (A to F) Scanning electron microscope (SEM) images presented the internal microstructure and different mineralization levels of CS-FS scaffolds in each group. In addition, the attached schematic diagram shows the structure changes of each group. (G) High-resolution transmission electron microscopy (HRTEM) images showed the pattern of the deposited CS produced by vacuum-induced mineralization on the FS collagen fibers. The CS particles which are evenly distributed along the collagen fibers were observed. (H) Energy spectrum analysis confirmed that the particles observed in HRTEM images were CS. Image Credit: Han, F et al., Bioactive Materials
Observations
The ultimate failure load of 51.65 ± 11.34 N of the 0.5CS-FS group was considerably higher than that of the polyethylene terephthalate (PET) and blank groups 12 weeks after the operation in the failure mode analysis, although there was no difference between the 0FS group and the 0.5CS-FS group.
The 0.5CS-FS group's ultimate stress was 7.30 ± 1.60 MPa, which was much greater than the other three groups. Furthermore, the stiffness of 10.88 ± 2.37 N/mm of the 0.5CS-FS group was much greater than that of the PET and blank groups, but no significant difference existed between the 0.5CS-FS and 0FS groups.
Although the CS-FS group's biomechanical attributes were still not identical to a normal rabbit rotator cuff, the 0.5CS-FS group achieved 73.2% of the final failure load of a conventional rotator cuff. The 0.5CS-FS group achieved 55.3% of normal rotator cuff stiffness. Dense inorganic particles with a diameter of 40.43 nm were attached to the surface of collagen fibers and wrapped evenly around them.
The proposed FS-based scaffolds maintained good tensile strength of 125.05 MPa and toughness of 14.16 MJ/m3, which were 1.93 and 2.72 times that of the natural tendon, respectively. Furthermore, CS-FS exhibited a wide range of bioactivities by boosting the differentiation and phenotypic maintenance of numerous types of cells involved in the tendon-bone junction composition. CS-FS played a critical role in tendon-bone interface regeneration and biomechanical function in both rat and rabbit RCT models, which could be done via the activation of the BMP-2/Smad/Runx2 pathway in bone marrow mesenchymal stem cells (BMSCs).
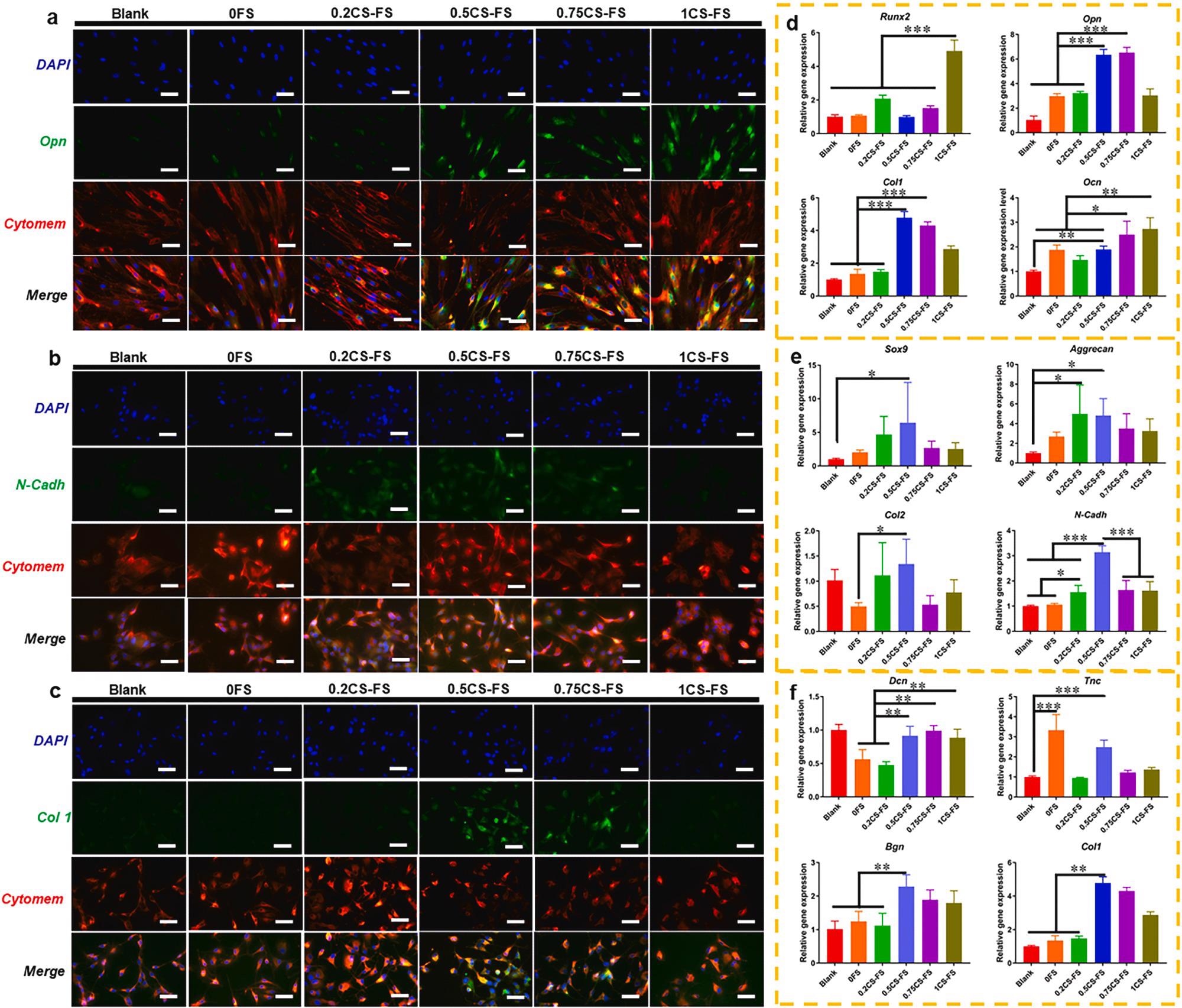
The CLSM images of the specific markers and Q-PCR analysis of specific gene expression for BMSCs, Chondrocytes and TSPCs cultured on CS-FS scaffolds in each group. The CLSM images showed the fluorescence labelled (A) Opn in BMSCs, (B) N-cadh in Chondrocytes and (C) Col 1 in TSPCs. Bar 100 μm (D) Expression of osteogenic differentiation-related genes in BMSCs cultured on each CS-FS scaffold. (E) Expression of chondrocytes phenotype-related genes in Chondrocytes cultured on each CS-FS scaffold. (F) Expression of tenogenic differentiation-related genes in TSPCs cultured on each CS-FS scaffold. CS deposition improved the bioactivity of FS scaffolds which promoted cell differentiation of several tissue cells. Image Credit: Han, F et al., Bioactive Materials
Conclusions
In conclusion, this study proposed that CS deposited on FS acts as a "bioactivating factor" to "bioactivate" FS, which allows it to repair various organs and transitional tissues. Fish scales were chosen as biomaterials for tendon repair. CS-FS was capable of considerably boosting cell differentiation and phenotypic maintenance after being modified by CS NPs for numerous types of cells generated from bone-interface-tendon, chondrocyte, BMSCs, and tendon stem/progenitor cells (TSPCs). In vivo, CS-FS improved transitional tissue and rotator cuff tendon between the bone and tendon healing, demonstrating that inorganic ions, specifically the combination of Si and Ca ions, had a more suitable integrated effect on promoting bone-interface-tendon repair.
According to the findings, natural fish scales combined with bioactive ions provide a novel class of high-performing biomaterials for repairing complicated tissues. The authors believe that as a result of their excellent strength and bioactivity, natural fish scale-based biomaterials are a promising candidate for therapeutic tendon restoration.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Han, F., Li, T., Li, M., et al. Nano-calcium silicate mineralized fish scale scaffolds for enhancing tendon-bone healing. Bioactive Materials 20 29-40 (2022). https://www.sciencedirect.com/science/article/pii/S2452199X22002079