Reviewed by Alex SmithJun 7 2022
Tohoku University researchers have discovered a way to keep rechargeable batteries’ metallic structure intact by stabilizing lithium or sodium depositions. The breakthrough inhibits battery degradation and short-circuiting, paving the way for higher-energy metal-anode batteries.
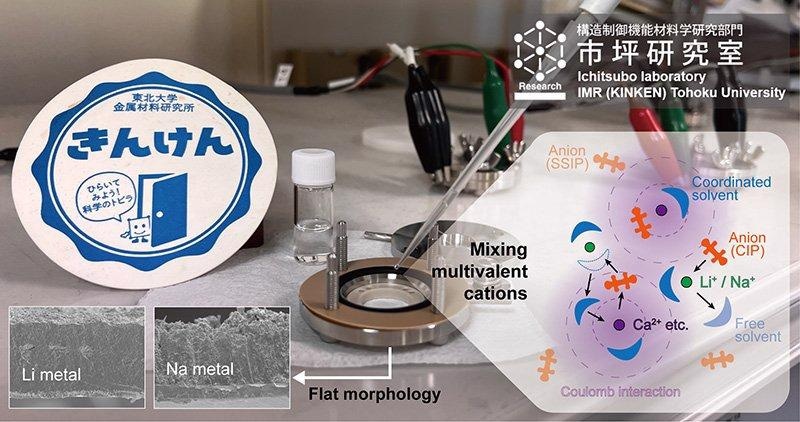 Multivalent cation additives modify the solvation structure of lithium or sodium cations in electrolytes and contribute to flat electrodeposition morphology. Image Credit: ©Hongyi Li.
Multivalent cation additives modify the solvation structure of lithium or sodium cations in electrolytes and contribute to flat electrodeposition morphology. Image Credit: ©Hongyi Li.
To meet the energy needs in a sustainable way, researchers are constantly working on developing safer, higher-capacity, and faster-charging rechargeable batteries. Metal anodes have the best chance of achieving that goal. However, using alkali metals has a lot of disadvantages.
When charging a rechargeable battery, ions flow from the cathode to the anode, and when producing power, they flow in the opposite direction. The structures of lithium and sodium are deformed by repeated metal deposition and dissolution.
Diffusion and electric field fluctuations in the electrolytes near the electrode surface also result in the formation of needle-like microstructures known as dendrites. Short-circuiting and decreased cycle capacity occur because the dendrites are weakly bonded and detach from the electrodes.
To overcome this issue, a group headed by Hongyi Li and Tetsu Ichitsubo from Tohoku University’s Institute for Materials Research introduced multivalent cations, like calcium ions, to the electrolyte, which changed and strengthened the solvation structure of lithium or sodium ions.
Our modified structure moderates the reduction of lithium or sodium ions on the electrode surface and enables a stable diffusion and electric field.
Hongyi Li, Institute for Materials Research, Tohoku University
The electrodeposited metals’ structure is preserved by the stabilized ions.
On May 20th, 2022, the results of their investigation were published in the journal Cell Reports Physical Science.
Li and Ichitsubo intend to improve the metal anodes’ interfacial design in the future to improve the battery’s cycle life and power density.
Journal Reference:
Li, H., et al. (2022) Dendrite-free alkali metal electrodeposition from contact-ion-pair state induced by mixing alkaline earth cation. Cell Reports Physical Science. doi.org/10.1016/j.xcrp.2022.100907.