Reviewed by Mila PereraSep 16 2022
Researchers recently discovered a reliable electrochemical method that simultaneously produces pure hydrogen from wastewater and removes refractory organic contaminants.
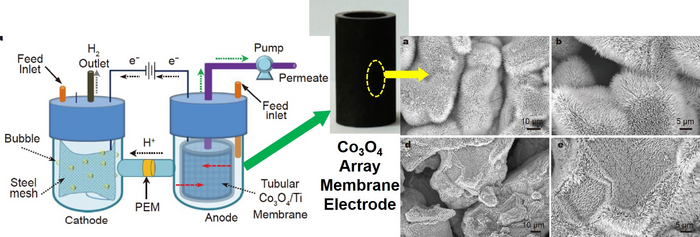 Schematic illustration of H-type PEM-ECMR with PEM separator and Ti-based membrane anode (Left); Photo of the tubular Co3O4/Ti membrane electrode (Middle); SEM images of the Co3O4 nanoarrays (Right). Image Credit: ©Science China Press
Schematic illustration of H-type PEM-ECMR with PEM separator and Ti-based membrane anode (Left); Photo of the tubular Co3O4/Ti membrane electrode (Middle); SEM images of the Co3O4 nanoarrays (Right). Image Credit: ©Science China Press
Professors Zhen Yin (College of Chemical Engineering and Materials Science, Tianjin University of Science and Technology), Jianxin Li (State Key Laboratory of Separation Membranes and Membrane Processes, Tiangong University), and Ding Ma (College of Chemistry and Molecular Engineering, Peking University) headed the recent research.
Co3O4 nanoneedle arrays, Co3O4 NNs, and the titanium membrane were used to build an effective and stable membrane anode, Co3O4 NNs/Ti membrane electrode, which was used to electrochemically oxidize refractory organic pollutants while simultaneously producing high-purity hydrogen over the cathode in wastewater under neutral conditions.
The assembly of 1D structures made from Co3O4 nanoparticles (NPs) and anchored on the membrane surface through an in-situ growth process without binders or additives resulted in the construction of the membrane electrodes with 3D array nanostructures, which significantly facilitated the electrochemical reactions.
The Co3O4 3D nanoarray may significantly improve the close interfacial adhesion with substrates, making it easier to transfer electrons and charges between the electrode and electrolyte, creating multiple catalytic active sites.
The loading of a 3D nanoarray on the surface of a membrane pore and using a flow-through configuration enabled the elimination of the diffusion/mass transfer restriction for conventional plate electrodes.
For wastewater treatment during the EAOP, the Co3O4 NNs/Ti membrane electrode demonstrated superior decontamination effectiveness and outstanding stability. The surface electric field distribution of membrane electrodes with various catalytic nanostructures was examined using a finite element simulation approach using the COMSOL Multiphysics.
To investigate the catalytic sites of the Co3O4 NNs/Ti membrane electrode in an electric field, in situ Raman studies were carried out in Na2SO4 solution.
The permeate solution analyses also explored the phenol degradation mechanism with the Co3O4 NNs/Ti membrane electrode.
The researchers developed an H-type ECMR (PEM-ECMR) to produce high-purity hydrogen, using a Nafion membrane as a separator and a Co3O4 NNs/Ti anode to show better phenol and dye water degradation capabilities, stable pure hydrogen production, and outstanding long-term stability.
The current work reveals that the PEM-ECMR cell can produce pure hydrogen and treat decentralized wastewater in a low electric field.
It would be a promising alternative route for simultaneous clean fresh water recovery and sustainable hydrogen energy production, whose energy cost can be further reduced if it powered with renewable energy in the future, such as solar energy. Moreover, the wastewater from industrial effluents can be used as hydrogen source via water electrocatalysis combined with pollutant electrooxidation process.
Zhen Yin, Professor, College of Chemical Engineering and Materials Science, Tianjin University of Science and Technology
Future research on electrochemical processes, modular decentralized water treatment systems, and technological breakthroughs for the treatment of contaminated water in conjunction with the production of renewable energy will be influenced by this work.
Journal Reference
Yin, Z., et al. (2022) Catalytic membrane electrode with Co3O4 nanoarrays for simultaneous recovery of water and generation of hydrogen from wastewater. Science China Materials. doi.org/10.1007/s40843-022-2168-y.