Reviewed by Mila PereraSep 21 2022
Professors Shaodong Zhou and Xiao-Nan Wu of the College of Chemical and Biological Engineering at Zhejiang University and the Department of Chemistry at Fudan University, respectively, are the principal investigators on the project outlined below.
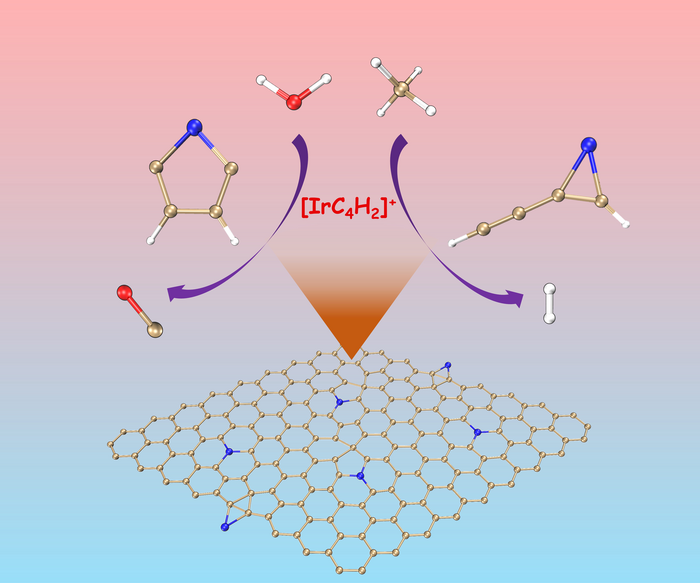 One of the two isomers of [IrC4H2]+ only activates methane while the other is solely reactive with water to produce CO. Image Credit: Science China Press
One of the two isomers of [IrC4H2]+ only activates methane while the other is solely reactive with water to produce CO. Image Credit: Science China Press
The studies were conducted utilizing a laser vaporization supersonic expansion ion-equipped ion trap mass spectrometer in conjunction with quantum chemistry calculations.
In the circumstances used, two isomers of [IrC4H2]+ co-exist with different reactivity levels; one only reacts with methane, while the other only reacts with water to produce carbon monoxide (CO).
When the coordination patterns are changed, the Ir center has relatively unique capacities for mediating the bond-making and bond-breaking processes.
While the π-aromaticity of the reaction complex is essential for the conversion of water, the reactivity toward methane mostly depends on the orbital orientation.
Due to the complex surface structure and composition of catalysts, it is of great significance to correlate the electronic structure of the active center with its reactivities. As an ideal model to study the reaction mechanism at strictly a molecular level, gas-phase reaction can be carried out under the conditions excluding the interference of the external environment and, being well repeatable.
Shaodong Zhou, Professor, College of Chemical and Biological Engineering, Zhejiang University
Zhou added, “Combined with quantum chemical calculation, it can help us understand deeply the reaction mechanism and implement the rational design of high-performance catalysts.”
Some design considerations for IR catalysts for methane steam reforming include:
- For the initial H3C-H activation, there will likely be more electron holes on the d orbits (likely caused by local polarization).
- In the interaction of the Ir center with the CH4/H2O molecule, an increase of the local aromaticity disfavors further transformation, whereas an increase of the local anti-aromaticity is indicative of further bond activation; the increase in both aromaticity and anti-aromaticity may be more noticeable for water.
Journal Reference
Yuan, B., et al. (2022) On the distinct reactivity of two isomers of [IrC4H2]+ toward methane and water. Science China Chemistry. doi:10.1007/s11426-022-1342-4.