 By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Oct 11 2022
By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Oct 11 2022In an article recently published in the open-access journal Scientific Reports, researchers discussed acrylic resin preparation and characterization using bioactive glasses.

Study: Preparation and characterization of acrylic resins with bioactive glasses. Image Credit: Cat Us/Shutterstock.com
Background
Although new substitutes have emerged, acrylic materials are still most frequently utilized in prosthetic dentistry to create removable dentures. Unfortunately, acrylic resins have significant drawbacks in addition to their many benefits, such as the restricted saliva flow in the denture base area. The factors that may contribute to dental alterations in the remaining teeth include wearing dentures, eating, and the consequent reduction in saliva flow in the region of the remaining teeth. As a result, acrylic resins ought to be altered to boost their bioactivity.
The incorporation of different kinds of nanoparticles, including titanium oxide and silver, is one of the methods being used to change acrylic polymers and create bioactive materials. The inclusion of various types of pharmaceuticals is another way. In addition to these methods, acrylic materials can also be modified through the application of various bioactive glasses.
This approach is already widely used in orthodontic adhesives, composite fillings, and glass ionomer cement. These glasses have so far been employed successfully in toothpaste, composites, and glass ionomer cement or Biomin. Fluorine ions can be released from glass by adding CaF2. However, too much CaF2 can cause crystalline phases to spontaneously crystallize. Chlorapatite will transform into hydroxyapatite (HA) in the presence of water from a dental standpoint. One type of fluorine glass used in these experiments is Biomin F.
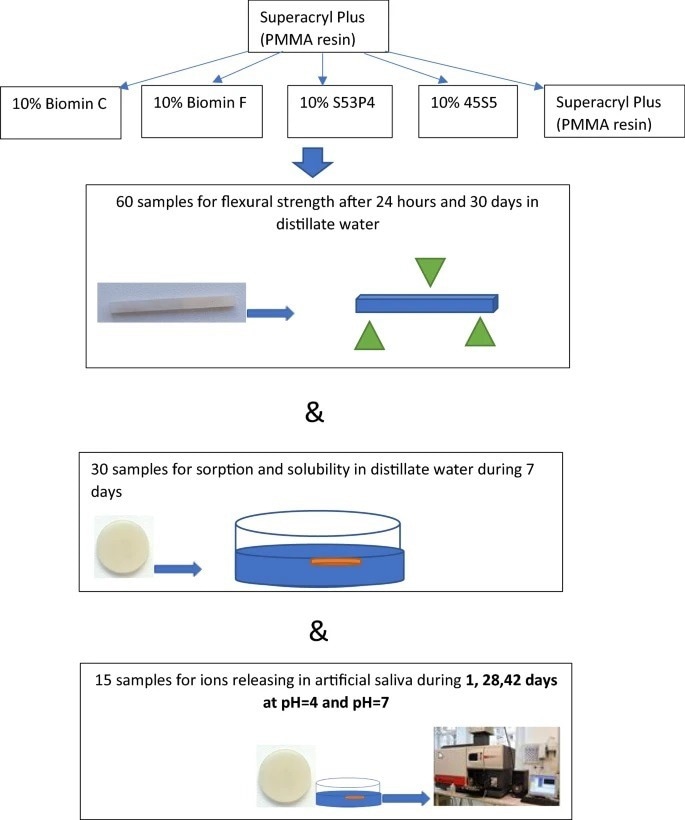
Scheme of sample preparation and testing. Image Credit: Raszewski, Z et al., Scientific Reports
About the Study
In this study, the authors used various varieties of glasses to create a bioactive acrylic material. 10% of four different types of powdered glasses were combined with commercially available polymerized acrylic resin, and subsequently, the mixture was dried. The samples' sorption capacity, flexural strength, and solubility were evaluated in accordance with ISO 20795-1:2013. A total of 60 samples were utilized in the experiments. Elution of the materials was undertaken for 0, 1, 28, and 42 days after they were immersed in artificial saliva with a pH of 4 to 7.
The team used inductively coupled plasma atomic emission spectrometry to evaluate the samples to find Ca, P, and Si ions, while ion chromatography was used to find F ions. While meeting the standards, the materials produced after modification with glasses had a lower compressive strength than pure polymethyl methacrylate. Two varieties of glass demonstrated solubility values that were higher than the value specified by the ISO standard. After 42 days in artificial saliva, Biomin C and S53P4 released P, Ca, and Si ions, respectively. Under acidic conditions for 28 and 42 days, S53P4 glasses and acrylic resins treated with 10% Biomin C could be useful sources of Ca and P ions.
The researchers created an acrylic substance that released calcium, phosphorus, and fluorine ions and had bioactivmethacrylate. It could produce a material with bioactive qualities based on analogous systems of methacrylate resins present in composite materials.
Observations
The results showed that all samples with bioactive glasses only had a 10% drop in flexural strength. The addition of particular kinds of glasses to poly (methyl) g glass S53P4 and Biomin C showed the maximum flexural strength. In comparison to the data obtained after 24 hours, a greater decrease in flexural strength was seen after 30 days. The minimum ISO standard requirement for denture base materials was 65 MPa, and all results were higher than this value. The acrylic system could release ions into the reaction environment when bioactive glass was added, which acted as a source of raw materials for the formation of HA. The type of glass being used affected how quickly ions were released.
After being altered using bioactive glass, the acrylic material created in this study met the ISO 20795-1: 2013 standard for sorption and flexural strength. The calcium and silicon phosphor ions in the acrylic resin made with a 10% addition of various active glasses could be released. The release of fluorine ions in an acidic environment was extremely dynamic in the case of Biomin F glass as it occurred within the first 24 h. Ions were released progressively over a 42-day period in a neutral atmosphere.
Under acidic conditions for 42 days, acrylic resins treated with 10% Biomin C and S53P4 glasses were useful sources of calcium cations and phosphate anions. In comparison to the unmodified resin, acrylic resins displayed a lower flexural strength with the addition of four different bioactive glasses. The sample containing Biomin F had a flexural strength of 78.05 ± 5.91 MPa, which was comparable to the sample containing pure PMMA. The range of heat-curing acrylic polymers' sorption in deionized water or synthetic saline was 17.5 ± 0.88 to 27.25 ± 1.04 μg/mm3. The sorption value for the reference material Superacryl Plus was 10 μg/mm3, and the sorption value for the Biomin F sample was 9.48 ± 0.81 μg/mm3.
Conclusions
In conclusion, this study discussed the development of composite materials made by combining PMMA with four distinct kinds of glasses. The four varieties of glasses gradually hydrolyzed under the action of water-released ions into the environment.
The team observed that any acrylic material's sorption and solubility were greatly influenced by its chemical structure, such as the presence of different ions. At an acidic pH, ions were released more quickly. At pH 7, more phosphate and silicate anions were released as a result of the equivalent salts forming. After 42 days at pH 4, the samples containing S53P4 glass produced 24.05 ± 3.61, 2.43 ± 0.36, and 3.02 ± 0.21 mg/L of PO43-, Ca2+, and SiO42-, respectively. At pH 4, the initial 24-hour release of fluorine ions was 7.05 ± 1.06 mg/L.
References
Raszewski, Z., Chojnacka, K., Mikulewicz, M., et al. Preparation and characterization of acrylic resins with bioactive glasses. Scientific Reports, 12, 16624 (2022). https://www.nature.com/articles/s41598-022-20840-1
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.