Reviewed by Mila PereraOct 28 2022
Although there is a rapidly growing market for rechargeable batteries, the essential raw materials are limited. Sodium-ion batteries, for example, could be a substitute. A collaborative research team from Helmholtz-Zentrum Berlin (HZB) and Humboldt-Universität zu Berlin has explored new groupings of electrode materials and electrolyte solutions for this purpose.
With operando techniques, it is possible to observe how solvated ions embed themselves in batterie electrodes. This might help to develop alternative batteries. Image Credit: © G. A. Ferrero
In contrast to lithium-ion batteries, which are based on the storage of lithium ions in the positive and negative electrodes of the battery, we are working on the one hand with sodium ions, as they also occur in cheap table salt. On the other hand, we store the sodium ions together with their solvate shell, i.e. solvent molecules from the electrolyte solution that separate the two electrodes. This makes it possible to realize completely new storage reactions.
Professor Philipp Adelhelm, Study Lead Author, Humboldt University
Professor Adelhelm directed the research group “operando battery analysis,” which was cooperatively founded by HZB and Humboldt University in 2020.
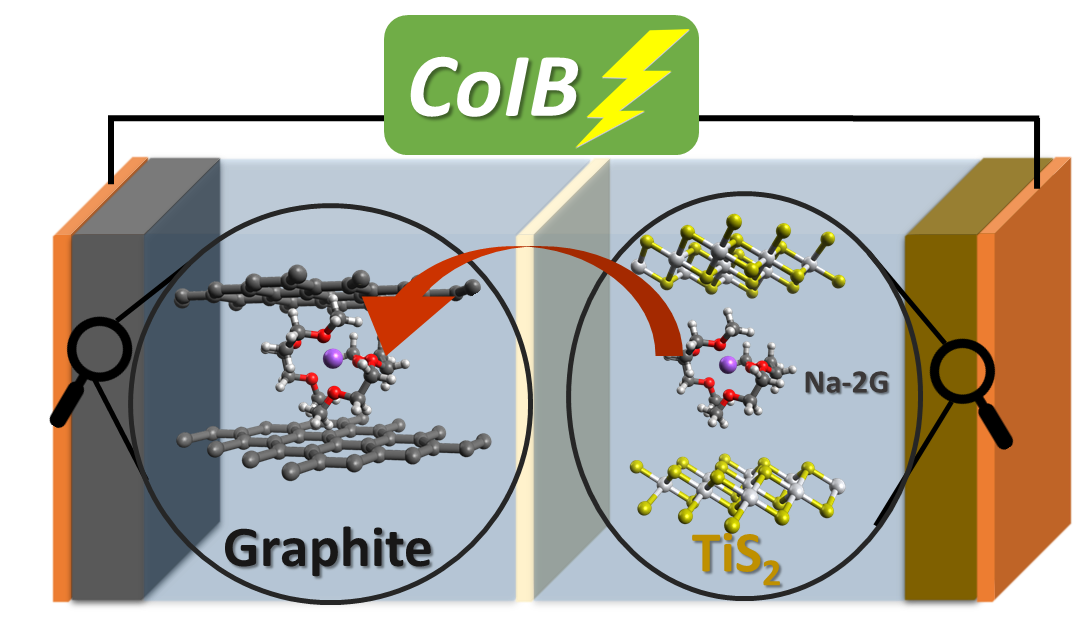
Co-intercalation is the storage of ions when joined by their solvation shell in a crystal lattice. Until now, this idea was restricted to the negative electrode of the sodium-ion battery.
The scientists working with Adelhelm have successfully expanded the concept to include the battery’s positive electrode.
With titanium disulphide and graphite, we have for the first time combined two materials that absorb and release the same solvent during charging and discharging of the battery.
Dr Guillermo A. Ferrero, Study First Author, Humboldt University
The researchers observed variations in the material during charging and discharging through operando measurements conducted in the X-Ray Core Lab at HZB on the LIMAX 160. This helped them to allocate the co-intercalation mechanism within the battery. They then applied this new insight to achieve a battery with two electrodes that depend on reversible co-intercalation of solvent molecules.
We are still in the early stages of understanding the implications of the co-intercalation batteries. But there are a few possible advantages we can envision. The process of co-intercalation could improve upon efficiency by enabling better low-temperature performance. It could also be utilised to improve upon alternative cell concepts such as using multi-valent ions instead of Li+ or Na+ storage that are particularly sensitive to the solvation shell.
Dr. Katherine A. Mazzio, Researcher, Helmholtz-Zentrum Berlin
This study was funded by the European Research Council (ERC) under the European Union's Horizon 2020 research and innovation program (grant agreement No. [864698], SEED).