Researchers from the Industrial Sustainable Chemistry group, under the direction of Prof. Gert-Jan Gruter, have made a significant advancement toward the creation of rigid, fully biobased polyesters in a recent study published in Nature Communications.
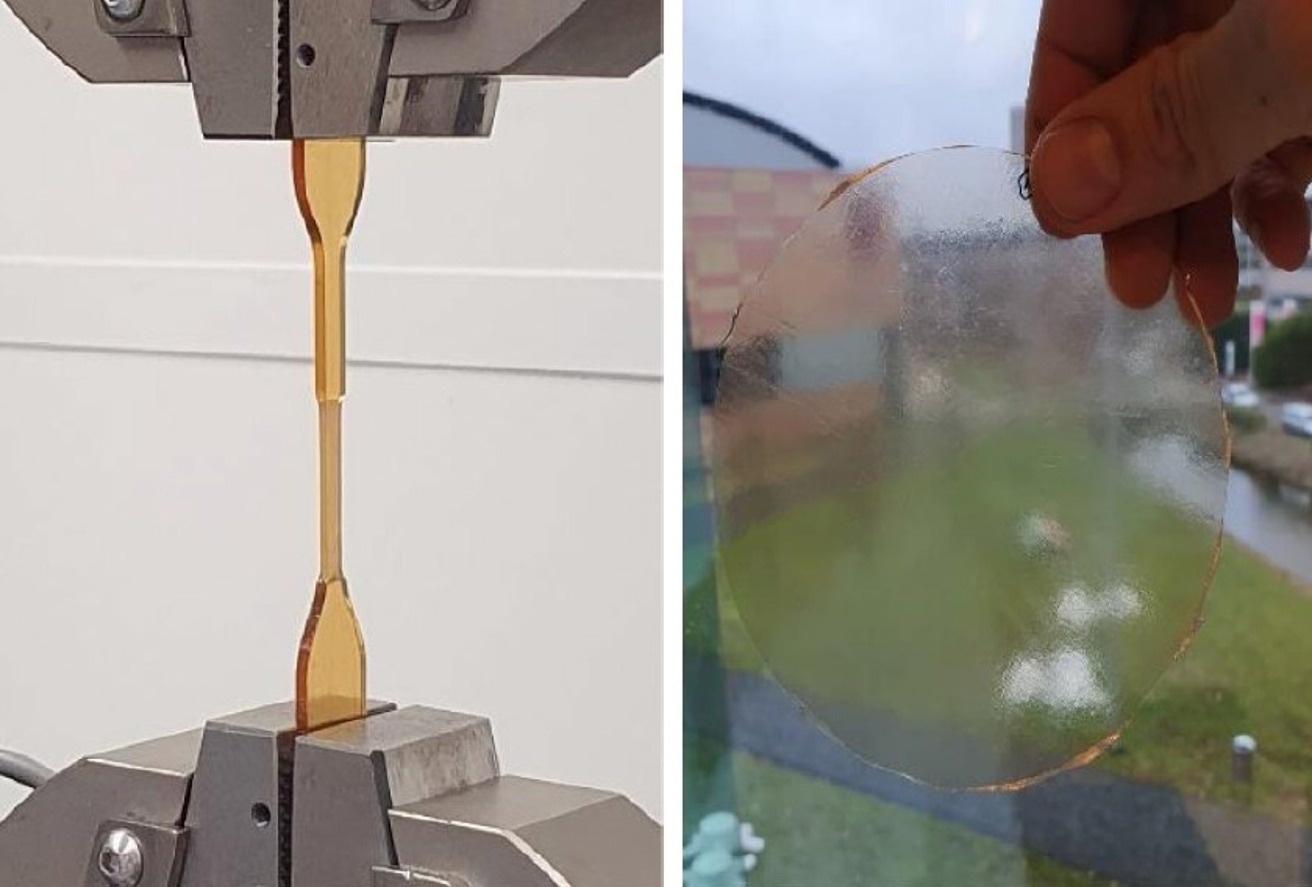 Tensile testing of the developed bio-based polymer (left) and a sample of polymer film (right). Image Credit: HIMS
Tensile testing of the developed bio-based polymer (left) and a sample of polymer film (right). Image Credit: HIMS
To get around the naturally low reactivity of biobased secondary diols and create polyesters with excellent mechanical and thermal properties and high molecular weights, they present a straightforward yet creative synthesis strategy.
With this approach, it is possible to assemble biobased plastics, which are extremely robust and long-lasting, from components that are already on the market.
The study detailed in Nature Communications was conducted as part of the RIBIPOL project, which was supported by the Dutch Research Council NWO and included funding from industry partners, including LEGO and Avantium.
The toy company gave the project its support as part of its efforts to find non-fossil replacements for its plastic bricks. Applications for bottles and films are appealing to Avantium.
The study’s first author is Daniel Weinland, a Ph.D. student who graduated on October 27th, 2022. The RIBIPOL project involves a total of five Ph.D. students, two of whom just recently successfully defended their theses.
Rigidity is Key
In general, small dialcohol and diacid molecules are used to create polyester plastics. These monomers are joined together in a condensation reaction, producing a lengthy polymer chain of alternating molecular building blocks.
The number of building blocks that make up the polymer chain and the inherent qualities of the monomers both contribute to the macroscopic material properties.
Their rigidity, in particular, is essential for a solid, robust, and long-lasting plastic. The dialcohol isosorbide made from glucose stands out among potential biobased monomers in this regard. It is already commercially available and has a very rigid molecular structure.
Due to isosorbide’s low reactivity, it has been difficult to produce useful polyesters based on isosorbide over the past 20 years. To obtain long enough polymer chains (to achieve a certain ductility), it was nearly impossible to do so while incorporating high enough amounts of isosorbide (to arrive at a strong and durable material).
Incorporating an Aryl Alcohol
By including aryl alcohol in the polymerization process, Weinland and his RIBIPOL colleagues have broken through this impasse.
As a result, reactive aryl esters are formed in situ, and the end group reactivity is significantly increased during polycondensation, the final step in the synthesis of polyester, where isosorbide’s low reactivity prevents chain growth in conventional melt polyesterification.
As a result, high fractions of the biobased, rigid secondary diol—even up to 100 mol%—could be incorporated to create high molecular weight materials.
Using isosorbide and succinic acid to create polyester, it was possible to create high molecular weight poly (isosorbide succinate) for the first time.
The resulting robust plastics outperform current plastics like PET in heat resistance, which is important for reuse. Additionally, the polymers based on isosorbide exhibit promising mechanical and barrier qualities that can outperform those found in typical fossil-based materials.
The study describes a novel polymerization strategy characterized by operational simplicity and the use of conventional polyester synthesis machinery.
The researchers envision exploring previously inaccessible polyester compositions based on monomers with a low reactivity as well as applying similar techniques in other classes of polymers like polyamides and polycarbonates. It is suitable for both existing and novel polyester compositions.
Journal Reference:
Weinland, D. H., et al. (2022) Overcoming the low reactivity of biobased, secondary diols in polyester synthesis. Nature Communications. doi:10.1038/s41467-022-34840-2.