Strong, non-biodegradable plastic, known as Nylon-6, cannot be recycled in the traditional sense. A team from the USA has recently revealed a new technique in the journal Angewandte Chemie.
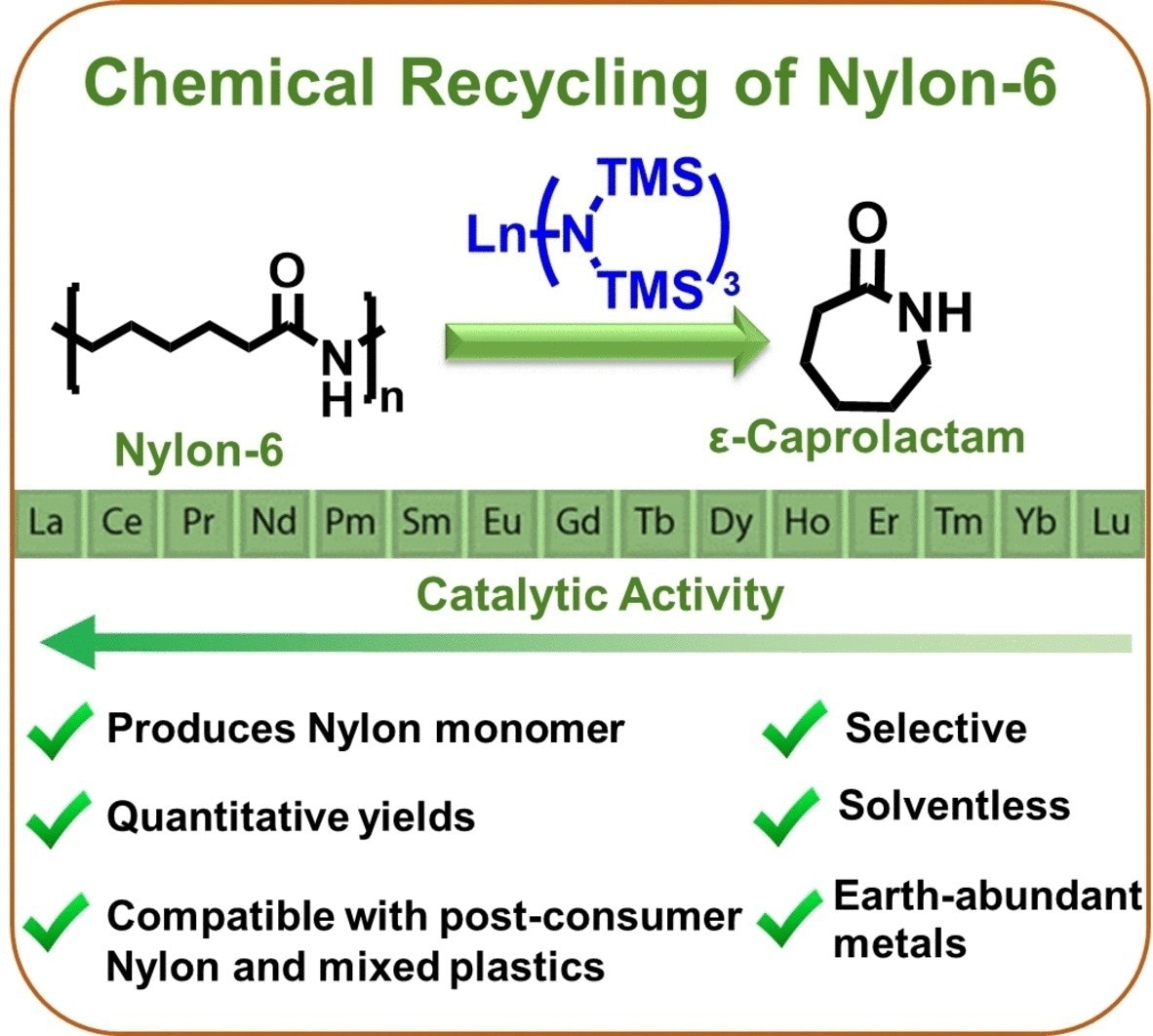
Image Credit: Wiley-VCH, Angewandte Chemie
Nylon-6 can be depolymerized selectively, nearly quantitatively, with no solvent, and at moderate temperatures to recover the monomer, ε-caprolactam, using a readily available lanthanum trisamido catalyst. Similar to unthreading pearls from a chain, the monomers are taken out of one end of the polymer one at a time.
Stockings are typically made of nylon fabric. Manufacturing automobiles, packaging, infrastructure, textiles, and fishing are just a few industries where it is the preferred material.
Its beneficial qualities, including elasticity, chemical resistance, high tensile strength, and high abrasion resistance, prevent it from degrading naturally. For instance, about 10% of the plastic waste in the oceans is leftover nylon fishing nets.
On an industrial scale, 5 million tons of ε-caprolactam are polymerized using the ring-opening method each year to create the variant Nylon-6. By 2026, the market is anticipated to generate 21.5 billion dollars. The threat to the environment and human health is growing in tandem with the growth of trash mounds.
Nylon-6 manufacturing also produces a significant amount of CO2. A pricey, multi-step process is used to create the monomer, ε-caprolactam, from raw materials derived from fossil fuels. Reclamation would save energy, reduce production costs, and conserve resources. Therefore, there is a high demand for Nylon-6 in a circular economy.
Nylon-6 is extremely challenging to recycle, whereas it is slowly picking up some other plastics. It cannot be melted to take on a new shape because the high temperatures needed cause it to partially decompose.
It also cannot be burned to produce energy because it produces harmful byproducts such as hydrogen cyanide. Previous techniques for recycling chemicals are either too complicated and ineffective or require questionable chemicals.
A new, effective catalytic process for recycling Nylon-6 has been created by a team led by Yosi Kratish and Tobin J. Marks from Northwestern University in Evanston, USA, and the National Renewable Energy Laboratory in Golden, USA.
With over 95% selectivity and over 90% yield, Nylon-6 is depolymerized to ε-caprolactam at the comparably low temperature of 240 °C without using solvents or hazardous chemicals. Polyethylene, Polypropylene, or Polyethylene Terephthalate mixtures do not, however, cause any interference.
The catalyst developed by the team, based on commercially available trisamido complexes of rare earth metals, is crucial to their success. The most active catalyst was a lanthanum complex. Calculations and experimental data point to a novel mechanism for the reaction.
The catalyst is covalently attached to the polymer, and a hydrogen ion is removed from a terminal amide N-H bond in the first step. Then, in a backbiting procedure, the ε-caprolactam units are separated from the chain’s end one by one.
Journal Reference
Wursthorn, L., et al. (2022) Selective Lanthanide-Organic Catalyzed Depolymerization of Nylon-6 to ϵ-Caprolactam. Angewandte Chemie. doi:10.1002/anie.202212543.