Using a home-built time-of-flight mass spectrometer (TOFMS), a research team led by Prof. Haiyang Li from the Dalian Institute of Chemical Physics (DICP) of the Chinese Academy of Sciences (CAS) has discovered a new competition mechanism in vacuum ultraviolet photoionization of dichloromethane that is useful for studying the stratospheric ozone depletion mechanism and photodegradation of harmful haloalkanes.
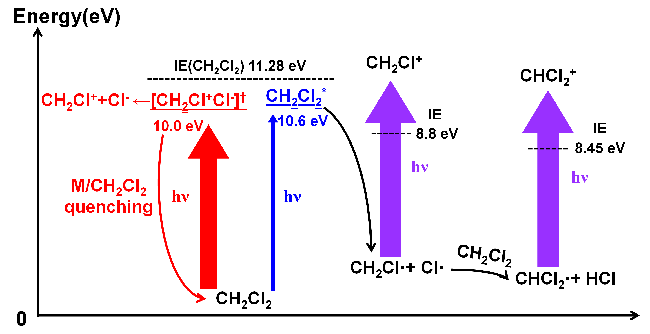 Proposed mechanism for the generation of CH2Cl+ and CHCl2+ when the neutral CH2Cl2 molecule was irradiated by a VUV Kr lamp. Image Credit: Yi Yu
Proposed mechanism for the generation of CH2Cl+ and CHCl2+ when the neutral CH2Cl2 molecule was irradiated by a VUV Kr lamp. Image Credit: Yi Yu
On January 31st, 2023, the study was published in The Journal of Physical Chemistry Letters.
Dichloromethane (CH2Cl2) is often employed as a feedstock for the synthesis of other chemicals, a reaction medium in the pharmaceutical sector, and an industrial solvent. Due to its low boiling point and high volatility, CH2Cl2 can be hazardous to the environment and human health.
The generation of ozone-depleting Cl atom can be stimulated by the strong vacuum ultraviolet (VUV) radiation present in the solar emission spectrum; as a result, the photochemistry of CH2Cl2 is essential to stratospheric ozone chemistry.
In this investigation, the researchers have demonstrated how CH2Cl2 gets photoionized when exposed to 10.0 and 10.6 eV light from a VUV krypton (Kr) lamp.
They showed that photoinduced ion pair and photodissociation-assisted photoionization were two competing pathways that created CH2Cl+ (PD-PI). At high CH2Cl2 number density, the ion-pair channel was effectively quenched, lowering its contribution.
Additionally, they demonstrated that CH2Cl2’s predominant photodissociation pathway was CH2Cl2 + hv → CH2Cl + Cl and that the Cl radical that resulted from this reaction could then interact with another CH2Cl2 molecule to produce the CHCl2 radical.
The photoionization of CHCl2+ then produced CHCl2+. For the quantitative description of the production efficiencies of CH2Cl+ and CHCl2+, they finally derived kinetic equations.
Our study enhances the overall understanding of the complicated photoexcitation behaviors of CH2Cl2 in the VUV regime, which helps to study the atmospheric photochemical process of haloalkanes and provides guidance for the photodegradation of hazardous haloalkanes.
Haiyang Li, Professor, Dalian Institute of Chemical Physics, Chinese Academy of Sciences
Li added, “Our study proposed new insights into the complicated photoexcitation behaviors of CH2Cl2 in the VUV regime and revealed the important role of photodissociation in VUV photoionization at low photon flux.”
The National Natural Science Foundation of China, the Scientific Instrument Developing Project of CAS, and DICP provided funding for this study.
Journal Reference:
Yu, Y., et al. (2023) Ionization of Dichloromethane by a Vacuum Ultraviolet Krypton Lamp: Competition Between Photoinduced Ion-Pair and Photodissociation-Assisted Photoionization. The Journal of Physical Chemistry Letters. doi:10.1021/acs.jpclett.2c03572.