Channeling ions into defined pathways in perovskite materials increases the stability and operational performance of perovskite solar cells, according to scientists. The discovery sets the stage for a new generation of solar cell technologies that are lighter, more flexible, and more efficient.
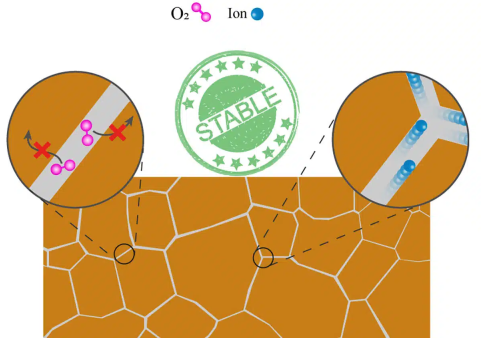
Image Credit: North Carolina State University.
Perovskite materials, which are identified by their crystalline structure, absorb more light than silicon. This suggests that perovskite solar cells can be thinner and lighter than silicon solar cells.
That opens the door to a host of new technologies, such as flexible, lightweight solar cells, or layered solar cells (known as tandems) that can be far more efficient than the solar harvesting technology used today in so-called solar farms. There’s interest in integrating perovskite materials into silicon solar cell technologies, which would improve their efficiency from 25% to 40% while also making use of existing infrastructure.
Aram Amassian, Study Corresponding Author and Professor, Materials Science and Engineering, North Carolina State University
Moreover, working with perovskite materials is difficult because long-term operational stability in perovskite solar cells has yet to be achieved.
Perovskites are ionic materials, which means that when a voltage is applied to them, ions move through them. These relocating ions are thought to contribute to chemical and structural changes in the material, making it inefficient and unstable. To make practical perovskite solar cells, scientists must solve this problem.
We have not found a way to prevent ions from moving through perovskite materials, but we have found that it is possible to steer these ions into a safe conduit that does not impair the material’s structural integrity or performance. It’s a big step forward.
Aram Amassian, Study Corresponding Author and Professor, Materials Science and Engineering, North Carolina State University
In this case, the safe conduit is referred to as a grain boundary. Perovskite materials have multiple crystal structures. When a perovskite is “grown,” it forms as a series of crystals—or “grains”—that are flush with each other. These grains are in charge of absorbing light and producing the charges that cause an electrical current.
Each of those grains has the same crystalline structure, but they are oriented slightly differently. A grain boundary is an area where two grains come together.
What we’ve found is that grains are better protected from impairment when the ions move predominantly along the grain boundary. Coupling this with what is already known about perovskite materials, it’s clear that problems start when grain boundaries are weak, which makes it easier for ions to move into the grains themselves.
Masoud Ghasemi, Study First Author and Co-Corresponding Author, Postdoctoral Researcher, Penn State
Masoud Ghasemi is a former postdoctoral researcher at NC State.
Masoud Ghasemi adds, “Designing stronger grain boundaries that protect the grains is essential to block migrating ions and other harmful species like oxygen from entering the grains, mitigating problematic chemical and structural changes in the material.”
Amassian elaborates, “This is an important insight because there are established techniques we can use to engineer perovskite materials and their grain boundaries; we can now make use of these approaches to protect the grains. We demonstrate how those techniques strengthen grain boundaries in this paper. In short, we now know what needs to be done to make far more stable perovskites.”
The research could also help to develop more effective energy storage technologies.
Amassian concludes, “This work advances our fundamental understanding of how ions move through any crystalline material that can carry charge, not just halide perovskites. We’re excited to talk to colleagues who work on energy storage about how this may inform the engineering of faster ion conductors.”
The study was carried out with funding from the US Office of Naval Research, under grant number N00014-20-1-2573; the National Science Foundation, under grant number CHE-1848278; and the US Department of Energy’s Office of Energy Efficiency and Renewable Energy under grant number DE-EE0009364.
Journal Reference
Ghasemi, M., et al. (2023) A multiscale ion diffusion framework sheds light on the diffusion–stability–hysteresis nexus in metal halide perovskites. Nature Materials. doi.org/10.1038/s41563-023-01488-2.