Lithium-ion batteries are utilized in a variety of applications, including not only small consumer electronics such as cell phones and laptop computers, but also electric vehicles, electric bicycles, and long-term power storage. Lithium-ion batteries can catch fire, which is uncommon, due to the instability of the liquid electrolytes in the battery construction.
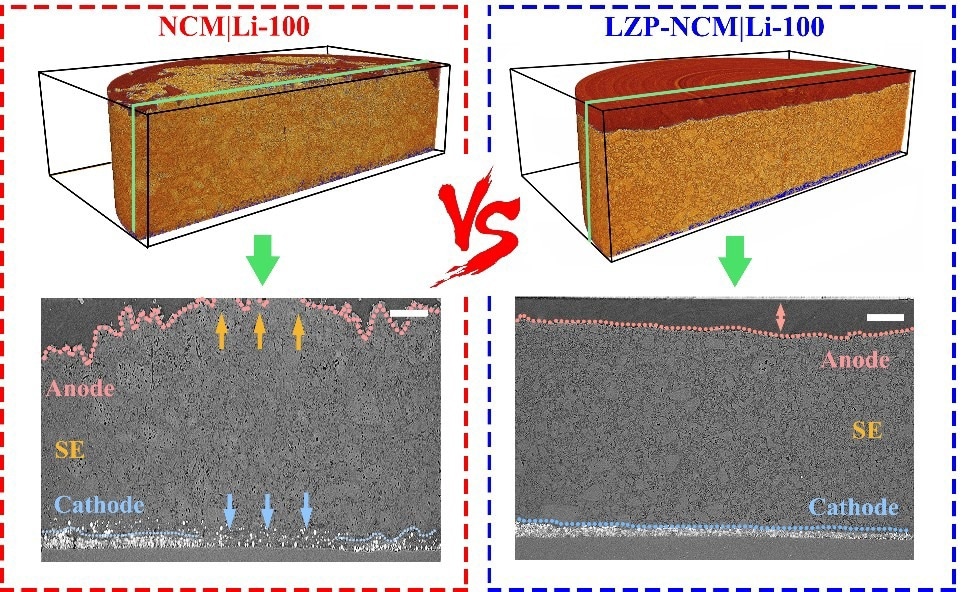 The comparison of 3D volume renderings and cross-sectional SXCT images of cycled NCM|Li and LZP-NCM|Li all-solid-state batteries. Image Credit: Yue Zheng, Fu Sun, and Jun Ma.
The comparison of 3D volume renderings and cross-sectional SXCT images of cycled NCM|Li and LZP-NCM|Li all-solid-state batteries. Image Credit: Yue Zheng, Fu Sun, and Jun Ma.
All solid-state lithium metal batteries, which have greater safety and energy density than robust lithium-ion batteries, are a viable option, but they degrade quickly.
A recent study headed by researchers from the Chinese Academy of Sciences’ Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) explains why this deterioration occurs and puts forth a simple, efficient solution.
On March 13th, 2023, the work was published in Science Bulletin.
Previously reported laboratory solid-state lithium metal battery prototypes exhibit fast performance decay that challenges their practical application. Revealing the fundamental science towards the problem and gaining an in-depth understanding of the underlying degradation mechanisms of all solid-state lithium metal batteries becomes crucially important for further development.
Guanglei Cui, Study Co-Corresponding Author, Professor and Group Leader, Solid Energy System Technology Center, Qingdao Institute of Bioenergy and Bioprocess Technology
The researchers utilized an imaging technology known as synchrotron X-Ray computed tomography (SXCT) to determine how these batteries degrade over time. SXCT scans generate highly detailed, three-dimensional images of objects without causing any damage to them.
The researchers investigated the batteries with NCM (LiNi0.8Co0.1Mn0.1O2) cathode active material, LPSC1 (Li6PS5Cl) solid electrolyte and lithium metal anode, called NCM|Li. The batteries were then SXCT scanned after zero cycles, 50 cycles, and 100 cycles.
The scientists found a breakdown between the two electrodes caused by uneven changes in lithium-ion flux by evaluating images of the cathode and anode after 100 cycles. Cracked particles of NCM were breaking off the cathode and becoming isolated.
The electric current was then focussed on the cathode’s remaining active areas. There was a larger possibility of lithium dendrite formation on the sections of the anode that faced the active NCM particles on the cathode. This could result in a variety of issues for batteries, including diminished capacity and safety.
For the first time, our work validates that inhomogeneous lithium-ion flux due to heterogeneous electrochemical reactions at the cathode zone dominates the exacerbated interfacial side restrictions and lithium dendrites at the lithium metal anode zone.
Dr Yue Zheng, Study First Author, Qingdao Institute of Bioenergy and Bioprocess Technology
“This can be effectively resolved by using a surface coating on the cathode material,” added Co-Corresponding Author Dr Jun Ma. The scientists coated the cathode with LiZr2(PO4)3 (LZP) and tested it again, scanning the battery with the surface coating after 100 cycles. The coated cathode batteries showed greater capacity retention and less capacity deterioration.
We hope this will open new avenues for research into the current limitations of solid-state batteries. Further development will focus upon the quantification of local current density in solid state batteries, the suppression strategy of anode lithium dendrite, strengthening of the internal contact of the cathode, and improving the critical current density.
Fu Sun, Study Co-Corresponding Author and Professor, Qingdao Institute of Bioenergy and Bioprocess Technology
Journal Reference:
Zheng, Y., et al. (2023). Codependent failure mechanisms between cathode and anode in solid state lithium metal batteries: mediated by uneven ion flux. Science Bulletin. doi.org/10.1016/j.scib.2023.03.021.