Zinc-air batteries are hailed as the technology of the future for various energy devices due to their potential high density, low cost, and eco-friendly design.
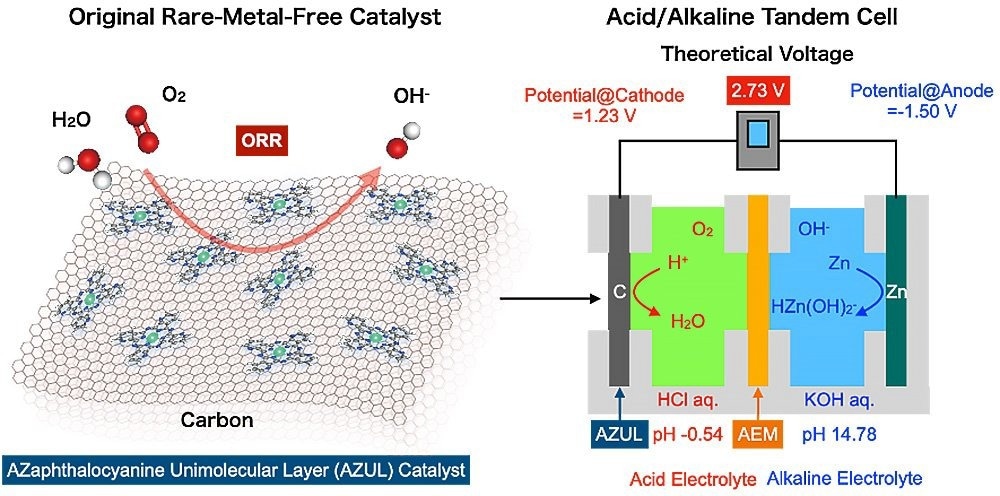 A schematic illustration of an original rare-metal free electrocatalyst (AZUL catalyst) and an acid/alkaline tandem cell. Image Credit: Hiroshi Yabu
A schematic illustration of an original rare-metal free electrocatalyst (AZUL catalyst) and an acid/alkaline tandem cell. Image Credit: Hiroshi Yabu
However, their low voltage of 1.4 V continues to prevent them from being used more widely, especially given that lithium-ion batteries can provide 3.7 V. Due to this drawback, zinc-air batteries are mostly used in low-power, long-lasting electronic devices like hearing aids.
If zinc-air batteries are used as the power source for cutting-edge gadgets like drones and electric vehicles, their drive voltage and output power density must be increased. A research team has recently succeeded in creating a zinc-air battery with an open circuit voltage of more than 2 V.
We harnessed a cell with a rare-metal-free cathode and acidic/alkaline electrolytes arranged in tandem to overcome the major bottleneck for metal-air batteries.
Hiroshi Yabu, Study Corresponding Author and Professor, Advanced Institute for Materials Research, Tohoku University
The potential difference between a battery’s cathode and anode determines the battery’s voltage. While the cathode potential in zinc-air batteries concerns the oxygen reduction reaction (ORR), which transforms oxygen’s chemical energy into electrical energy, the anode potential in zinc-air batteries includes zinc dissolving into the electrolyte.
Due to the potent alkaline electrolytes, zinc-air batteries, which are frequently used in hearing aids, potentially have a voltage of 1.9 V. Overvoltage is frequent, although the ORR is rare. As a result, the output voltage is around 1.4 V.
Due to the high ORR reaction activity of precious metal catalysts like platinum and carbon (Pt/C), several scientists have attempted to get around this. However, access to these resources is constrained and costly. The pH level of the electrolytes has a significant impact on the reaction potential at each electrode.
As per the Pourbaix diagram, which depicts pH on the horizontal axis and reaction potential on the vertical axis, the dissolution potential of zinc is lowest under alkaline circumstances, while the redox potential of oxygen is highest under acidic conditions.
Yabu added, “This led us to realize that by making the electrolyte on the zinc anode side alkaline, and creating acidic conditions on the cathode side, we could generate a higher voltage.”
Journal Reference:
Ishibashi, K., et al. (2023) Rare-metal-free Zn–air batteries with ultrahigh voltage and high power density achieved by iron azaphthalocyanine unimolecular layer (AZUL) electrocatalysts and acid/alkaline tandem aqueous electrolyte cells. APL Energy. doi:10.1063/5.0131602.