Around the world, at least 2 billion people regularly consume water containing pathogenic bacteria.
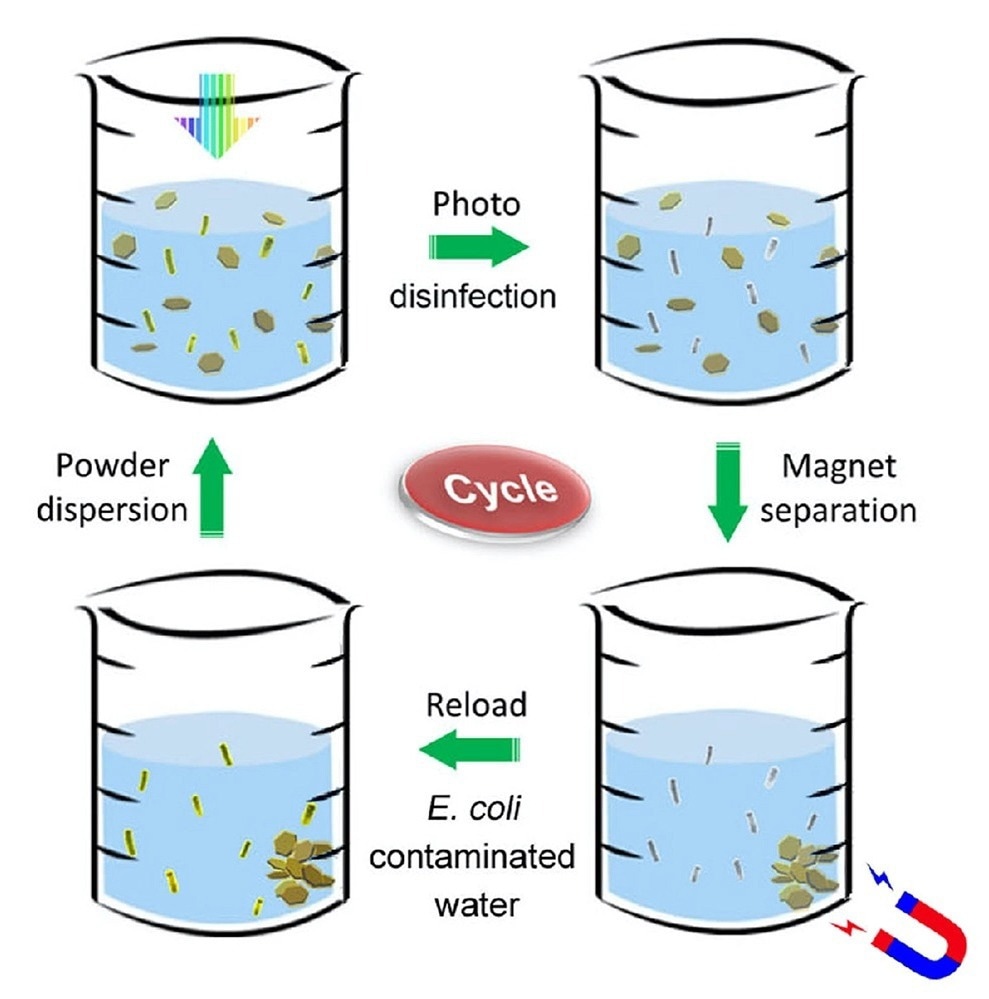 Disinfectant powder is stirred in bacteria-contaminated water (upper left). The mixture is exposed to sunlight, which rapidly kills all the bacteria (upper right). A magnet collects the metallic powder after disinfection (lower right). The powder is then reloaded into another beaker of contaminated water, and the disinfection process is repeated (lower left). Image Credit: Tong Wu/Stanford University
Disinfectant powder is stirred in bacteria-contaminated water (upper left). The mixture is exposed to sunlight, which rapidly kills all the bacteria (upper right). A magnet collects the metallic powder after disinfection (lower right). The powder is then reloaded into another beaker of contaminated water, and the disinfection process is repeated (lower left). Image Credit: Tong Wu/Stanford University
Now, researchers from Stanford University and the SLAC National Accelerator Laboratory have created a cheap, recyclable powder that, when exposed to regular sunlight, kills millions of water-based bacteria each second.
According to the Stanford and SLAC team, the discovery of an ultrafast disinfectant might represent a huge step forward for almost 30% of the world’s population who do not have access to clean drinking water. In a study published on May 18th, 2023 in Nature Water, their findings are reported.
Waterborne diseases are responsible for 2 million deaths annually, the majority in children under the age of 5. We believe that our novel technology will facilitate revolutionary changes in water disinfection and inspire more innovations in this exciting interdisciplinary field.
Tong Wu, Study Co-Lead Author and Former Postdoctoral Scholar, Materials Science and Engineering, Stanford School of Engineering, Stanford University
Chemicals, which can result in harmful byproducts, and UV light, which takes a while to disinfect and needs a power source, are two common water-treatment procedures.
A safe metallic powder created at Stanford University serves as a novel disinfectant by absorbing the sun’s UV and high-energy visible radiation. Copper, iron oxide, aluminum oxide, and molybdenum sulfide are all present as nanoscale flakes in the powder.
We only used a tiny amount of these materials. The materials are low cost and fairly abundant. The key innovation is that, when immersed in water, they all function together.
Yi Cui, Study Senior Author and Fortinet Founders Professor, Materials Science and Engineering and of Energy Science & Engineering, Stanford Doerr School of Sustainability, Stanford University
Fast, Nontoxic, and Recyclable
The molybdenum sulfide/copper catalyst functions as a semiconductor/metal junction after collecting photons from the sun, allowing the photons to dislodge electrons.
One of the most biologically harmful types of oxygen, hydroxyl radicals, is created when the released electrons react with the surrounding water. The newly generated chemicals swiftly destroy the bacteria by severely harming their cell membranes.
Approximately 1 million E. coli bacteria per milliliter (.03 oz) of room-temperature water was employed by the Stanford and SLAC team for the investigation.
We stirred the powder into the contaminated water. Then we carried out the disinfection test on the Stanford campus in real sunlight, and within 60 seconds no live bacteria were detected.
Bofei Liu, Study Co-Lead Author and Research Scientist at EEnotech Inc
According to him, the fine nanoflakes can move swiftly, come into touch with plenty of bacteria, and quickly destroy them.
Additionally, sunlight’s chemical byproducts vanish swiftly.
Cui added, “The lifetime of hydrogen peroxide and hydroxy radicals is very short. If they don’t immediately find bacteria to oxidize, the chemicals break down into water and oxygen and are discarded within seconds. So, you can drink the water right away.”
Additionally, recyclable is the nontoxic powder. Iron oxide makes it possible to detach the nanoflakes from water using a regular magnet. To clean 30 distinct samples of polluted water, the researchers in the study employed magnets to collect the same powder 30 times.
Cui further added, “For hikers and backpackers, I could envision carrying a tiny amount of powder and a small magnet. During the day you put the powder in water, shake it up a little bit under sunlight and within a minute you have drinkable water. You use the magnet to take out the particles for later use.”
He stated that the powder could be beneficial in wastewater treatment facilities that now disinfect treated water with UV lights.
“During the day the plant can use visible sunlight, which would work much faster than UV and would probably save energy. The nanoflakes are fairly easy to make and can be rapidly scaled up by the ton,” Cui concluded.
The study concentrated on E. coli, which can lead to severe gastroenteritis and can be fatal. The US Environmental Protection Agency has imposed a zero-tolerance standard for the presence of E. coli in drinking water.
The Stanford and SLAC team wants to test the new powder against additional water-borne pathogens, such as viruses, protozoa, and parasites that are also responsible for deadly diseases.
Bofei Liu is currently a research scientist at EEnotech Inc., a water purification company co-founded by Cui. Tong Wu is a professor at Tonji University in Shanghai.
Harold Y. Hwang, professor of applied physics in the School of Humanities and Sciences and professor of photon science at SLAC, and director of the Stanford Institute for Materials & Energy Sciences are the other Stanford co-authors.
Former engineering postdocs Chong Liu, Jiayu Wan, Feifei Shi, Ankun Yang, Kai Liu, and Zhiyu Lu; and former engineering PhD students Jie Zhao and Allen Pei also contributed to the study.
The US Department of Energy provided funding for the research.
Journal Reference:
Wu, T., et al. (2023) Solar-driven efficient heterogeneous subminute water disinfection nanosystem assembled with fingerprint MoS2. Nature Water. doi:10.1038/s44221-023-00079-4.