Lithium batteries play a pivotal role in powering devices, including phones, computers, cars, and various tools like drills and weedwhackers. However, as technology continues to advance, their current format may struggle to keep up.
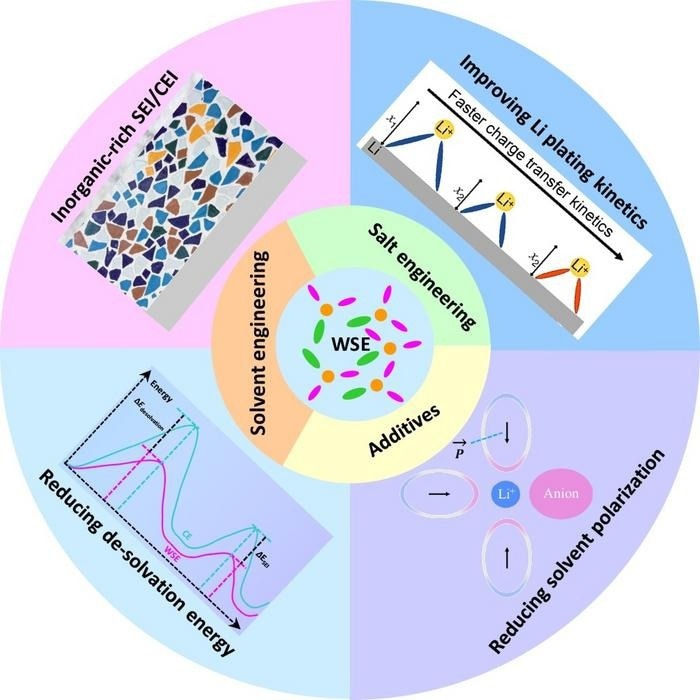
Emerging electrolytes with a different microstructure to conventional electrolytes may help advance lithium batteries to keep pace with future technologies, according to a review paper from researchers at the Hong Kong Polytechnic University. Their analysis concluded that better kinetics, reduced solvent polarization, reduced desolvation energy and more inorganic-rich interphases may improve lithium batteries. Image Credit: Energy Materials and Devices, Tsinghua University
Recent research carried out by researchers at Hong Kong Polytechnic University suggests a promising path forward: enhancing the development of the electrolytes responsible for energy storage and discharge.
Their findings were published in Energy Materials and Devices on September 18, 2023.
Lithium batteries have revolutionized our modern life. The performance of lithium batteries —including energy density, lifespan, safety, and more—is greatly determined by the recipe and microstructure of the electrolytes.
Zhijie Wang, Study First Author and Postdoctoral Research Fellow, Department of Applied Physics and the Research Institute of Smart Energy, Hong Kong Polytechnic University
Lithium batteries are primarily composed of two current collectors, a negative electrode, a positive electrode, an electrolyte, and a separator. The electrolyte's role is to transport positively charged lithium ions from the positive electrode, across the separator, to the negative electrode, while negatively charged ions move in the opposite direction.
As the lithium ions traverse the electrolyte, the anode accumulates free electrons, resulting in a positive charge at one of the current collectors. This positive current is discharged to supply power to a device, circulating through it and then returning to the battery as a negative current.
Typically, electrolytes are composed of a solution containing lithium salt in a polar solvent. This solvent is a substance that, due to the shape of its constituent molecules, produces a slight electrical charge, enabling it to dissolve the salts and release the lithium ions.
In traditional electrolytes, lithium ions separate from the salt anions. However, in the more recent development of (localized) high-concentration electrolytes, the salt anions are solvated by lithium ions. This unique arrangement serves specific functions aimed at enhancing battery performance.
However, Wang stated that a category of electrolytes known as weakly solvating electrolytes, which were initially suggested for this application three years ago, may present a route to more durable batteries. In this particular electrolyte type, the lithium ions form weak coordination with the solvent molecules and simultaneously coordinate with the negatively charged salt anions.
Such a structure can be obtained in a relatively dilute salt concentration and without the use of non-solvating diluters. This makes it different from conventional electrolytes and (localized) high-concentration electrolytes. It can improve the low-temperature, fast-charging, safety, and cycling properties of lithium batteries, so it has attracted intensive research interest in recent years.
Zhijie Wang, Study First Author and Postdoctoral Research Fellow, Department of Applied Physics and the Research Institute of Smart Energy, Hong Kong Polytechnic University
In their study, Wang and the corresponding author, Biao Zhang, conducted an analysis of recent research papers pertaining to weakly solvating electrolytes. They determined that there was a notable absence of fundamental design principles and future research directions in this area. Zhang serves as an associate professor in the Department of Applied Physics and the Research Institute of Smart Energy at Hong Kong Polytechnic University.
We found, from the current literature, that the key to constructing weakly solvating electrolytes is creating a balance between the interactions of lithium ions with solvent and anions. We also determined that the design concepts of weakly solvating electrolytes can be extended to other batteries, such as systems made with sodium, potassium, magnesium, or zinc.
Zhijie Wang, Study First Author and Postdoctoral Research Fellow, Department of Applied Physics and the Research Institute of Smart Energy, Hong Kong Polytechnic University
The researchers also suggested that forthcoming research endeavors in this field should prioritize streamlining the synthesis methods, enhancing production efficiency, and lowering the costs associated with the electrolyte components.
“The goal of this paper is to promote the understanding of both research and industrial communities on the functions, design principles, and recent research progress of the emerging weakly solvating electrolytes,” Wang notes. “We hope our insights contribute to developing next-generation lithium batteries with robust properties and thus boost their share of the power source market, especially in electric vehicles.”
The Hong Kong Polytechnic University provided support for this study.
Journal Reference
Wang, Z., & Zhang, B. (2023). Weakly solvating electrolytes for next-generation lithium batteries: design principles and recent advances. Energy Materials and Devices. doi.org/10.26599/emd.2023.9370003.