Zinc, which is inexpensive, abundant, and ecologically friendly, could be the answer to better batteries, but there is a huge issue: In terms of power output, aqueous zinc ion batteries (AZIBs) cannot compete with lithium-ion batteries. To see what electrode material composition could bring AZIBs up to par, a Chinese research team created two organic frameworks with the same elements but arranged differently.
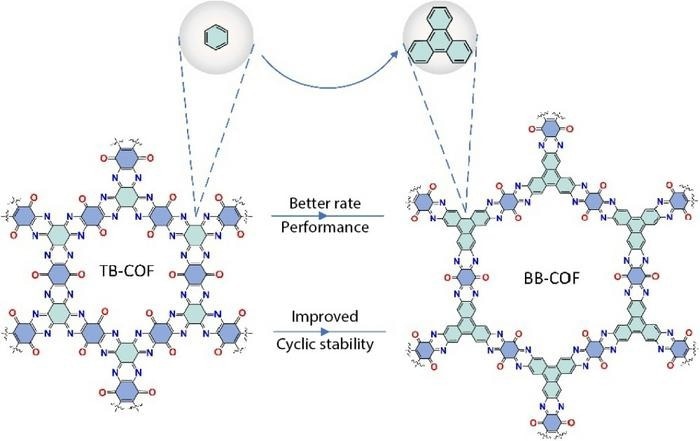
Researchers put two newly developed cathode materials head-to-head to determine how densities of active sites — where ions react to gain electrons and create a battery’s charge — affect electrochemical performance. The framework on the right has better electrochemical performance. Image Credit: Energy Materials and Devices, Tsinghua University Press
When tested, the structure with an appropriate density of active sites—where zinc ions gain electrons to recharge the battery—performed better.
On October 8th, 2023, the researchers published their findings in Energy Materials and Devices.
For over a decade, AZIBs have gained considerable attention as a highly promising battery technology. Various electrode materials have been explored, with inorganic options being extensively studied. However, these materials often encounter challenges, including crystal structure degradation and limited specific capacity.
Meilin Li, Study Co-First Author and Professor, Shenzhen Institute of Advanced Technology (SIAT), Chinese Academy of Sciences
The organic compounds that make up the cathode are molecules organized in a crystalline shape. During charge and discharge reactions, which correspond to the absorption and release of zinc ions, functional groups undergo alternating oxidation and reduction processes.
Ions react and pick up electrons in the active sites found in these compounds. However, the number of reactions that inorganic molecules can support and the length of time they can last before breaking down are both restricted.
Li added, “In contrast to inorganic materials, organic materials exhibit superior redox properties, high specific capacity and structural flexibility. In this paper, we designed two covalent organic framework (COF) materials with the same structure and number of energy groups to investigate the correlation between the densities of active sites and electrochemical performance.”
Whereas the second COF made use of triquinoxalinylene benzoquinone (TB-COF), the first COF employed the chemical compound benzoquinoxaline benzoquinone (BB-COF). There was the same amount of energy groups in each of the two ring-shaped frameworks. The carbon atoms bound to oxygen or nitrogen, known as active sites, are stored in the energy groups.
Additionally, BB-COF was bigger, with the energy groups positioned farther apart. Although the molecule BB-COF had more sparsely packed active sites in its energy groups, TB-COF was generally denser.
And according to Li, that seems to be the secret to improved electrochemical performance.
Li further added, “Although TB-COF boasts a commendable initial specific capacity stemming from its densely packed functional groups, its susceptibility to capacity deterioration due to potent interactions places it at a disadvantage when vying for the role of an AZIBs cathode material. BB-COF, on the other hand, demonstrated stable cycling even at -40 degrees Celsius for 2,000 cycles — meaning it could remain stable even as a battery charges and discharges 2,000 times.”
After examining the morphology and chemical composition of the COFs, the researchers concluded that BB-COF’s larger diameter allowed for faster ion transport and better use of the active sites.
After 10,000 cycles at room temperature, BB-COF maintained a specific capacity of 72 milliampere-hours per gram mass or the measurement of charge per weight. Per gram mass, the specific capacity of TB-COF decreased to 40 milliampere-hours.
“Regulating and controlling pore dimensions to obtain the optimal densities of active sites are believed to not only enhance the zinc ion transport rate in COF pathways but also to help maintain the stability of COF structures. Therefore, this approach is a direction for future efforts,” Li stated.
Fanbin Zeng, Senlin Li, Sanlue Hu, and Cuiping Han from SIAT, as well as Qingming Liu, Tengfei Zhang, and Jun Zhou from Towngas Energy Academy, are the study co-authors.
The study was supported by the Chinese Academy of Sciences' Special Research Assistant Funding Project, the Guangdong Basic and Applied Basic Research Foundation, the China Postdoctoral Science Foundation, the Outstanding Youth Basic Research Project of Shenzhen, and the National Natural Science Foundation of China.
Journal References
Li, M., et al. (2023) Unlocking high-performance organic cathodes: tailoring active group densities in covalent frameworks for aqueous zinc ion batteries. Energy Materials and Devices. doi:10.26599/EMD.2023.9370007.
Article Revisions
- Jan 6 2025 - Meta Description has been updated to fall inline with Google recommendations and a malformed link has been repaired.