NMR spectra are typically collected in solutions made up of deuterated solvents due to the fact that a protonated solvent will yield large solvent peaks which may hide the solute’s spectral features. In addition, many advanced NMR spectrometers use the deuterium signal of the solvent for locking and shimming.
Examining Reactions in Native Solution
Although using proton-free solvents is advantageous in many ways, there are situations demanding the analysis of reactions or products in their native solution. This can be achieved with the Magritek Spinsolve benchtop NMR spectrometer. The fast external lock of the Spinsolve benchtop NMR spectrometer avoids the need for deuterated solvents to achieve high-resolution performance. This article presents some representative illustrations of proton NMR spectra collected in completely protonated solvents.
Representative Examples
This example involved the preparation of two 200mM ibuprofen solutions in chloroform, one in a protonated solvent and second in deuterated solvent. The 1D Proton protocol’s Quickscan option was used to record the NMR spectra. The spectra for both samples are shown in Figure 1. Although there is overlapping of the solvent peak with solute peaks of 4 and 5, the Spinsolve benchtop NMR spectrometer clearly resolved all other spectral features.
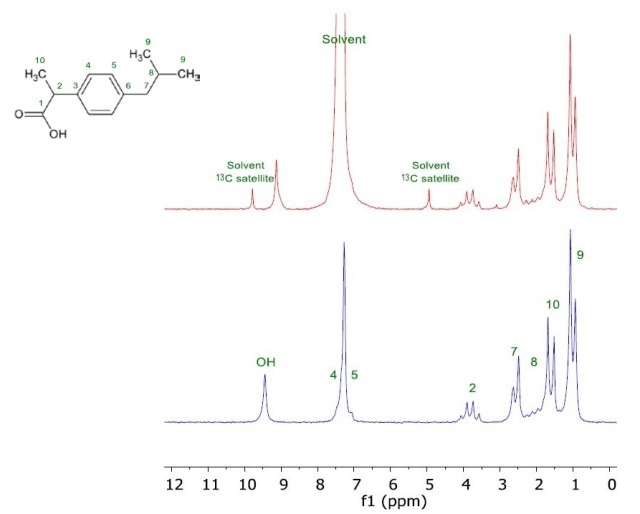
Figure 1. NMR spectrum of 200mM Ibuprofen in CHCl3 (red) and in CDCl3 (blue).
Figure 2 shows a second-year chemistry experiment involving the synthesis of p-nitroacetanilide by nitrating acetanilide.
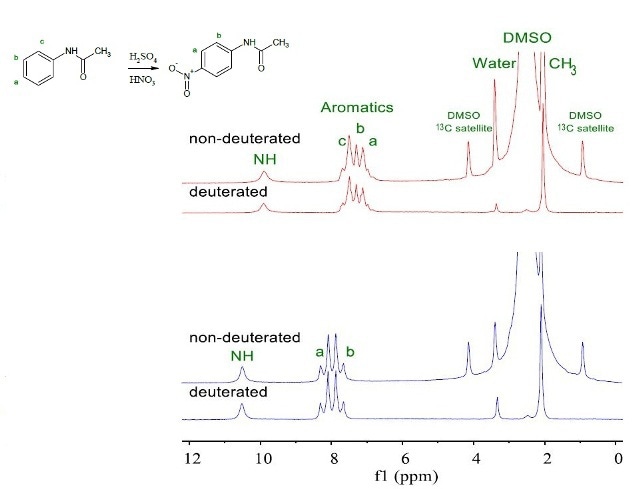
Figure 2. NMR spectra of 200mM acetanilide (red) and p-nitroacetanilide (blue) in deuterated and non-deuterated DMSO.
Figure 3 shows the proton NMR spectrum of a caffeine sample in water obtained from a chromatographic column for subsequent analysis on the Spinsolve benchtop NMR spectrometer. Further sample purification or processing was not required in this experiment.
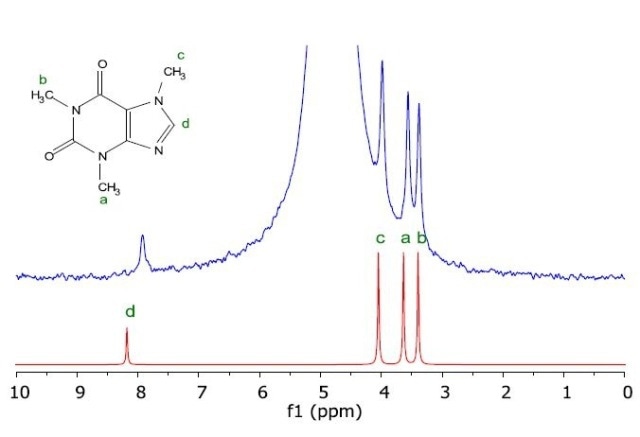
Figure 3. Measured NMR spectrum of caffeine in H2O (blue) as taken from a HPLC column, and the simulated NMR spectrum of the molecule (red).
Conclusion
The results clearly demonstrated the ability of the Spinsolve benchtop NMRspectrometer to collect proton NMR spectra both in deuterated and non-deuterated solvents. Although the solute’s peaks may be obscured by the large solvent peak when using non-deuterated solvents, these solvents allow studying reactions and reagents in their native solution. These problems and the cost of operation need to be considered when the benchtop NMR is used as an on-line monitoring instrument.
This information has been sourced, reviewed and adapted from materials provided by Magritek.
For more information on this source, please visit Magritek.