SamplerSight Pharma is a Windows-based software solution exhibiting validation capabilities and user-friendly features.
It effectively oversees syringe-operated samplers and gathers pertinent data from a LiQuilaz® II particle counter. This software empowers operators to handle sampling requirements in batch-oriented operations efficiently.
Beyond its operational control, SamplerSight Pharma offers a user-friendly interface presenting a comprehensive batch overview. Information is visually represented through histograms, time plots, and tabular data, ensuring ease of interpretation.
The software incorporates robust database filtering functionalities based on batch, lot, and location, facilitating swift responses to database queries and streamlined sample reporting.
The system further streamlines operations with multiple preconfigured or customizable sample configurations, minimizing setup time when transitioning between batches of diverse types.
Notably, the calibration of USP 788 sensors is a quick and straightforward process, typically accomplished within 20 to 30 minutes.
SamplerSight Pharma Software fully complies with 21 CFR Part 11 standards, providing reassurance in regulatory requirements. It also comes complete with IQ/OQ/PQ documentation. Optional services such as installation setup, validation, and training are available to cater to specific user needs.
Benefits
Cost Reduction
- A specialized application designed for batch monitoring minimizes integration time and expedites the reporting process
- Compatibility across multiple platforms enables seamless migration to new computer systems, enhancing overall system reliability
Easy to Use
- Intuitive icons facilitate effortless software operation
- Database filtering functions for batch/lot/location ensure swift responses to database queries and facilitate streamlined sample reporting
- Predefined reporting tools expedite batch reports, while user-configurable formats permit adjustments to align with site-specific requirements
Increased Productivity
- Immediate access to real-time data facilitates a swift response to particle-related issues.
- Including multiple user-defined sample configurations minimizes setup time when transitioning between batches of various types. This singular application supports a range of products, reducing training time for operators.
Features
- Diverse data presentations, including histograms, time plots, and tabular formats.
- Configuration and storage options specific to each sample, including setup, recipes, and storage.
- Batch and filters for efficient data retrieval and reporting.
- Flexible user-configurable identification labels for customization.
- Automatic printing functionality for each sample.
- Robust reporting capabilities with CSV and PDF file export for comprehensive documentation.
- Alarm specification and generation functionality to enhance operational awareness
- Manual control diagnostics screen for hands-on monitoring and adjustment.
Applications
Collecting, storing, and viewing data for:
- Release of injectable products
- Monitoring batches in liquid production
- Monitoring the cleanliness of medical devices
- Oversight of particulate matter in ophthalmic solutions
Source: Particle Measuring Systems
| . |
. |
| Sampling systems supported |
APSS-2000 with LiQuilaz® II liquid particle counters |
| On-line RS-485 sensors supported |
LiQuilaz II (E-Series and S-Series) |
| Minimum computer requirements |
Please refer to the Microsoft® operating system minimum requirements.
RS-232 serial communication port (when used in conjunction with non-Ethernet devices), network interface port |
| Software required |
Windows® 10 or Windows 7 with Microsoft SQL Server 2014 installed
(SQL Server Express is included with the software) |
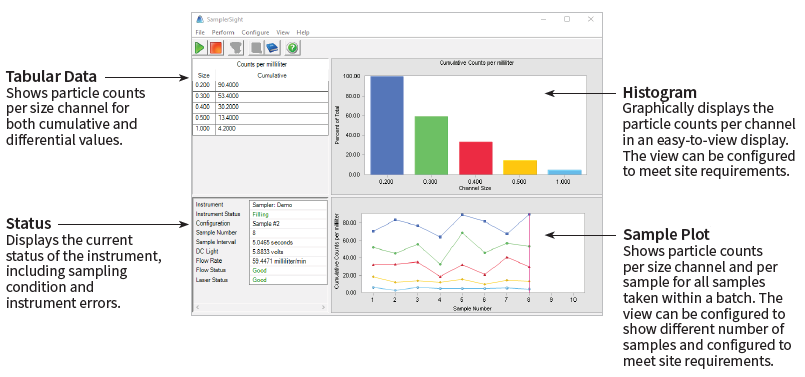
Image Credit: Particle Measuring Systems