Scientists at the National Institute of Standards and Technology (NIST), Sandia National Laboratories, College Park and the University of Maryland have developed a sequence of nanowire batteries to show that the electrolyte layer’s thickness can limit the size and performance of the battery.
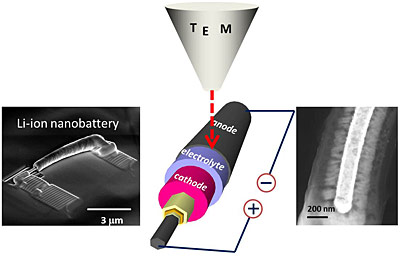 Using a transmission electron microscope, NIST reearchers were able to watch individual nanosized batteries with electrolytes of different thicknesses charge and discharge. The NIST team discovered that there is likely a lower limit to how thin an electrolyte layer can be made before it causes the battery to malfunction.
Using a transmission electron microscope, NIST reearchers were able to watch individual nanosized batteries with electrolytes of different thicknesses charge and discharge. The NIST team discovered that there is likely a lower limit to how thin an electrolyte layer can be made before it causes the battery to malfunction.
The findings are significant because battery performance and size play a major role in the advancement of autonomous MEMS, which can be used in a broad array of fields, including industrial and medicine monitoring. It is not possible to fabricate MEMS devices with sizes below a millimeter with existing battery technologies, which significantly affects the systems’ efficiency.
A research team comprising Alec Talin, a NIST scientist, and colleagues has fabricated a veritable forest of small solid-state lithium ion batteries with a height of 7 µm and a width of 800 nm to determine the smallest possible size of batteries that can be made of existing materials and study their performance.
The research team fabricated the tiny batteries by depositing layers of anode material, electrolyte, cathode material and metal with different thicknesses on silicon nanowires. Using a transmission electron microscope, the team monitored the current flow traversed by the batteries and the changes in their materials when they charged and discharged. The team discovered that when the electrolyte film’s thickness decreases below a threshold value of about 200 nm, the electrons can cross the electrolyte border rather than traversing the wire to the battery and then on to the cathode. Electrons follow a short cut via the electrolyte, creating a short circuit, which in turn breaks down the electrolyte leading to faster discharge of the battery.
Talin explained that although the reason causing the breakdown of the electrolyte is not clear, the researchers understand the importance of developing a novel electrolyte for designing smaller batteries. The major material, LiPON will not function at the thicknesses needed for fabrication of high-energy-density rechargeable batteries for MEMS.