Apr 7 2014
In medicine, light therapy is currently used to treat seasonal affective disorder, psoriasis, and other medical conditions, while highly targeted lasers may be used for specific skin disorders, eye diseases, or cancers. Advances in imaging methods and equipment now allow scientists to see the effects of light at the cellular level, leading to research on potentially transformative ways to use specific types of light for more even complex and direct manipulation of individual cells.
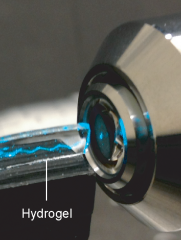 Laser beam reflecting within hydrogel slab. Credit: Seok-Hyun Yun, Harvard Medical School
Laser beam reflecting within hydrogel slab. Credit: Seok-Hyun Yun, Harvard Medical School
Optogenetics is a relatively new technique that harnesses light to activate or inhibit light-responsive proteins that control specific cell functions. Most optogenetics research to date has targeted brain cells, allowing scientists to manipulate individual neurons and observe the effects. While a groundbreaking tool for studying the inner workings of the brain without the need for electrodes or any direct contact with brain tissue, the challenge of shining a light deep within the body has limited broader uses of optogenetics.
For most proposed clinical uses, light needs to be delivered evenly across a number of cells to have a reproducible therapeutic effect, but human tissue is not transparent and scatters, absorbs, or otherwise reduces light penetration, reducing the ability to deliver light below the skin. To address this delivery challenge, NIBIB grantee Seok-Hyun Andy Yun, Ph.D., and researchers at Harvard Medical School and various institutions in Korea, experimented with using transparent hydrogels in combination with optogenetics.
Hydrogels are similar in concept to commonplace, edible gelatin, but are being researched for use in medical implants. Currently, most medical implants are made with rigid materials like plastic and metal. Though such implants are carefully designed to work within the body, by nature, placing a hard object among relatively soft tissues can cause inflammation and other unwanted side effects. In contrast, hydrogels can be easily constructed using more biologically-friendly (“biocompatible”) materials, and their high water content and flexible nature may conform more closely to muscles, organs, and other internal body parts so that light is guided efficiently. Some fluids can also flow through hydrogels, which may allow for different types of uses than can be achieved with implants made of conventional, impermeable materials.
Experimenting with the hydrogel recipe, Yun and colleagues devised a strong yet flexible, clear hydrogel slab able to guide a laser beam, bouncing the light back and forth within its boundaries. The researchers were also able to seed the hydrogel with cells—like fruit cocktail in a gelatin dessert—which refract and scatter light, creating a uniform glow throughout the slab.
When lit by a fiber optic and implanted just under the skin in mice, the glowing hydrogel could be clearly seen. In a follow-up experiment, the researchers grew cells that glow green in the presence of cadmium, a toxic heavy metal often used to make quantum dot sensors. When cadmium-core quantum dots were injected into mice with hydrogel implants, the cells within the hydrogel glowed green. However, when dots with a more biocompatible zinc-based coating were injected, the hydrogel did not glow, suggesting that the zinc coating effectively shielded the cells in the hydrogel from cadmium toxicity.
To test the hydrogel’s ability to deliver a treatment, the researchers created slabs embedded with cells that glow in the presence of calcium. The slabs were then fitted with a blue light fiber optic and implanted in mice with chemically induced diabetes. When exposed to blue light, a protein called melanopsin sets off a cascade of activity within the cells, including the release of calcium, that help to control the effects of diabetes.
In mice exposed to the blue light, the cells in the implanted hydrogel glowed much more compared to hydrogels in unexposed mice, suggesting the former group had higher levels of intracellular calcium and anti-diabetic activity. To further validate this finding, the mice were given a glucose tolerance test to see how long it took for their blood sugar levels to return to normal—since diabetes impairs the body’s ability to process sugar, blood sugar levels remain abnormally high for longer periods of time than in a person or animal without diabetes. The light-exposed diabetic mice achieved regular blood sugar levels within an hour and a half, while the untreated diabetic mice continued to have elevated blood sugar even after two hours, indicating that light delivered via the hydrogel produced a measurable biological effect and may someday be a useful means of delivering optogenetic treatments.
By delivering light inside the body in a controlled and predictable manner and being able to host genetically engineered cells that respond to light or emit light in response to specific chemical signals, the hydrogels created by Yun and colleagues may help address some of the challenges with using optogenetic approaches in clinical care.
“Further validation and refinement of this technology are needed before it can be applied to human health issues, but these findings are very promising for advancing the field of light-based medical treatments,” said Richard Conroy, Ph.D., who oversees NIBIB-supported research on new technologies for optical imaging.
Reference
Choi M, Choi JW, Kim S, Nizamoglu S, Hahn SK, Yun SH. Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo. Nat Photonics. 2013 Nov (7)12:987-994. doi:10.1038/nphoton.2013.278