May 5 2016
All living cells are essentially soft and squishy balloons containing DNA, proteins, and a large amount of water. These cells are surrounded by oily membranes, which can withstand considerable amounts of stress like bending, stretching, and squeezing. It was only recently that researchers have started to understand the functions and organization resulting from that stress.
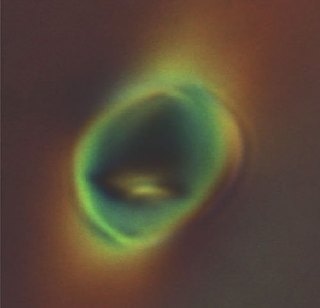 Microscopic image shows dramatic deformation of soft vesicles floating inside liquid crystals in the nematic phase. UW-MADISON MATERIALS RESEARCH SCIENCE AND ENGINEERING CENTER
Microscopic image shows dramatic deformation of soft vesicles floating inside liquid crystals in the nematic phase. UW-MADISON MATERIALS RESEARCH SCIENCE AND ENGINEERING CENTER
Taking a cue from this emerging insight, researchers at the Materials Research Science and Engineering Center (MRSEC) of the University of Wisconsin—Madison are attempting to reproduce features of those all-inclusive design principles in artificial systems containing complex fluids and simple membranes. The study shows that parameters, which were previously unappreciated, can mold soft materials including biological membranes. The results of the study have been reported in the journal, Proceedings of the National Academy of Sciences.
What we’re trying to do is take design principles in bacteria and see if we can translate them to synthetic systems. This is a model, trying to recreate some of the properties of bacteria to understand, in a simpler system, what’s going on.
Nicholas Abbott, MRSEC director, University of Wisconsin
The paper highlighted a major concept that strain in complex membranes and fluids can be shared in unexpected ways to regulate the properties and shape of soft materials. Earlier, researchers theorized that membrane strain plays an important role in how organisms regulate the compositions of varied areas on the surfaces of the cells.
Douglas Weibel, the paper’s co-author and a professor of biochemistry at UW–Madison, explored a mechanism on how elastic energies present in the membrane could transfer cellular components to the coiled ends of bacterial cells.
As a reference model, small synthetic shells known as vesicles were developed by the scientists. These vesicles contained materials that were identical to the membranes surrounding the living cells. Biological membranes were approximated by the tiny spheres without any of life’s intricate external decorations or internal machinery to confuse the outcomes. In order to stress, strain and squeeze the membrane spheres, the vesicles were suspended within a complex fluid known as liquid crystal. Liquid crystals, similar to those utilized in digital watch displays, can occur in varied states. Their components, like most liquids, freely move around in various directions. However, at particular electromagnetic conditions or temperatures, similar orientations are adopted by the molecules that make up liquid crystals. This eventually results in the so-called nematic phases, where they are all directed in the same direction.
According to studies performed in the past, objects floating in liquid crystals can affect a nematic phase’s molecular alignments. However, the resulting disruptions are not inertly accommodated by liquid crystals, and instead stiff objects are pushed back by these complex fluids.
However, no one knows what exactly would happen if a synthetic vesicle entered into the mix. The team noted that when the nematic phase is turned on, distortions occur in the floating spheres, but this effect was not seen in all the vesicles. Although the larger orbs remained round, the smaller orbs were flattened, pinched, and squeezed into extended shapes similar to American footballs.
In an effort to unravel the existing energetic mechanisms, the researchers sought the help of UW–Madison mathematics professor Saverio Spagnolie, who applied a novel numerical method to measure the forces that could contribute to the discrete deformation patterns. The team was surprised by the physics responsible for such shapes.
Usually when people think about membranes, the primary forces they consider are associated with elasticity. But it turns out that the bending stiffness has absolutely nothing to do with the shapes that we see in this work.
Saverio Spagnolie, Mathematics Professor, UW-Madison
A competition between elasticity and surface tension of the liquid crystal caused the distortion in the vesicles, irrespective of the flexibility or stiffness in cell membranes.
Going into the problem, there was no obvious reason to think that surface tension would be a relevant piece of the puzzle.
Saverio Spagnolie, Mathematics Professor, UW-Madison
In the future, the scientists are hoping to clarify the source of the surface tension existing in the system. They are also planning to study whether identical forces could shape the compositions of membranes that are made from combined components, just like the living cell’s surfaces.
The lead author of the paper is Peter S. Mushenheim, a former graduate student in Abbott’s laboratory. The study was supported by the National Science Foundation-funded MRSEC that brings together multidisciplinary scholars to address challenging issues in materials science.