Aug 4 2016
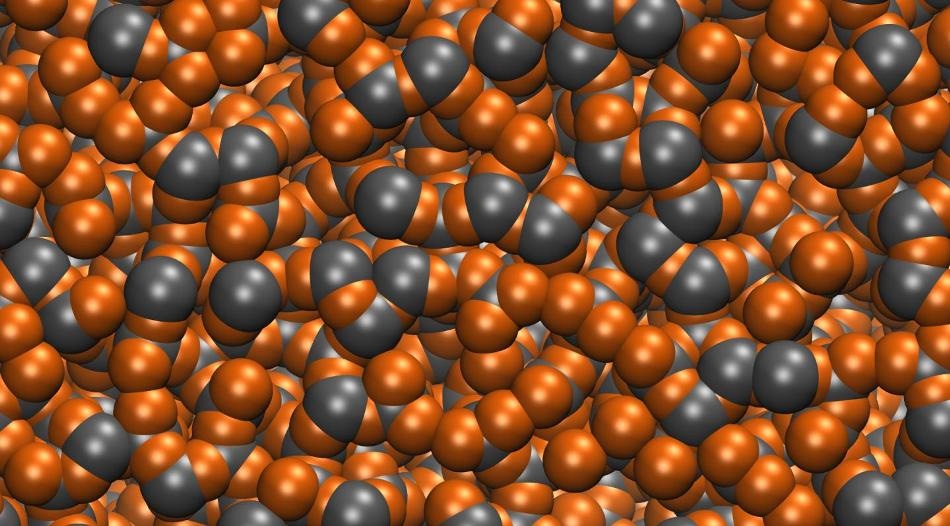 When metal alloys are melted, the atoms lose their ordered structure and become amphorous, as seen above. Most metals alloys will snap back to their rigid crystal structures when cooled back down but bulk metallic alloys will retain the random amorphous structure even in the solid state. (Image courtesy of the Vlassak Group/Harvard SEAS)
When metal alloys are melted, the atoms lose their ordered structure and become amphorous, as seen above. Most metals alloys will snap back to their rigid crystal structures when cooled back down but bulk metallic alloys will retain the random amorphous structure even in the solid state. (Image courtesy of the Vlassak Group/Harvard SEAS)
Action figures of the Man of Steel are rarely made from steel. These figures, like everything else that exists in everyday life, are made of plastic that is easy to mold and cost-effective.
Bulk metallic glasses are metallic alloys that comprise of an efficiently ordered atomic structure that can be changed into an amorphous, non-crystalline structure, providing the malleability of plastic to the metal while simultaneously maintaining its conductivity and durability.
Metallic glasses have a wide range of applications, such as golf clubs, medical industries, nuclear reactor engineering, and electronics.
These alloys, in addition to their different uses, are also considered to be complex and they often contain five or six different elements together with expensive noble metals such as palladium or gold.
Another issue is that scientists have no idea what combination of elements will develop them. The only way to identify if a metallic alloy is a bulk metallic glass is by synthesizing the alloy, followed by melting and quenching. The next step is to check whether it crystallizes. This process takes up a lot of time and is expensive.
Recently, a new method used to predict which alloys could develop from a bulk metallic glass was developed by researchers from the Harvard John A. Paulson School of Engineering and Applied Sciences (SEAS), together with colleagues from Duke and Yale universities.
Nature Communications features a description of this research.
For the first time, we’ve observed a strong correlation between the glass-forming ability of an alloy and properties that we can easily calculate ahead of time.
Joost J. Vlassak, Professor of Materials Engineering, SEAS
The atoms lose their ordered structure when metal alloys are melted. Almost all metal alloys are capable of snapping back to their firm crystal structures after being cooled. Cooling bulk metallic glasses at specific rates results in retaining the random amorphous structure even in the solid state.
Additional options are available for some alloys when their crystal structures are considered. The atoms of these alloys tend to crystallize in a variety of ways when these alloys are cooled into solids.
“If a particular alloy composition exhibits many structurally different, stable or metastable crystal phases that have similar formation energies, these phases will compete against each other during solidification,” Vlassak said. “Essentially, the liquid becomes so confused, it remains amorphous as it solidifies.”
When you get a lot of structures forming next to one another that are different but still have similar internal energies, you get a sort of frustration as the material tries to crystalize. The material can’t decide which crystalline structure it wants to converge to, and a metallic glass emerges. What we created is basically a measure of that confusion.
Eric Perim, Postdoctoral Researcher, Duke University
Duke University researchers created a database to simulate several hundreds of crystalline structures that a single alloy is capable of taking. The researchers developed a program to examine the different structures and then compare the required energy to create them.
Alloys that are considered to be the ideal candidates to develop a metallic glass are those that can develop an increasing variety of structures with similar energy.
The predictions were then experimentally verified by teams from Yale and Harvard. The new approach is capable of predicting the formation of known metallic glasses 73% of the time, and has detected several hundreds of new candidates for metallic glass produced from simple, two-element alloys.
With researchers now being able to predict good candidates for metallic glass, they can next focus on looking for new material systems.
This study was supported by the National Science Foundation. It was co-authored by Eric Perim, Dongwoo Lee, Yanhui Liu, Cormac Toher, Pan Gong, Yanglin Li, W. Neal Simmons, Ohad Levy, Joost J. Vlassak, Jan Schroers, and Stefano Curtarolo.