Oct 5 2016
 This image shows direct utilization of elemental sulfur in the synthesis of microporous polymers and its gas separation performance. Credit: KAIST
This image shows direct utilization of elemental sulfur in the synthesis of microporous polymers and its gas separation performance. Credit: KAIST
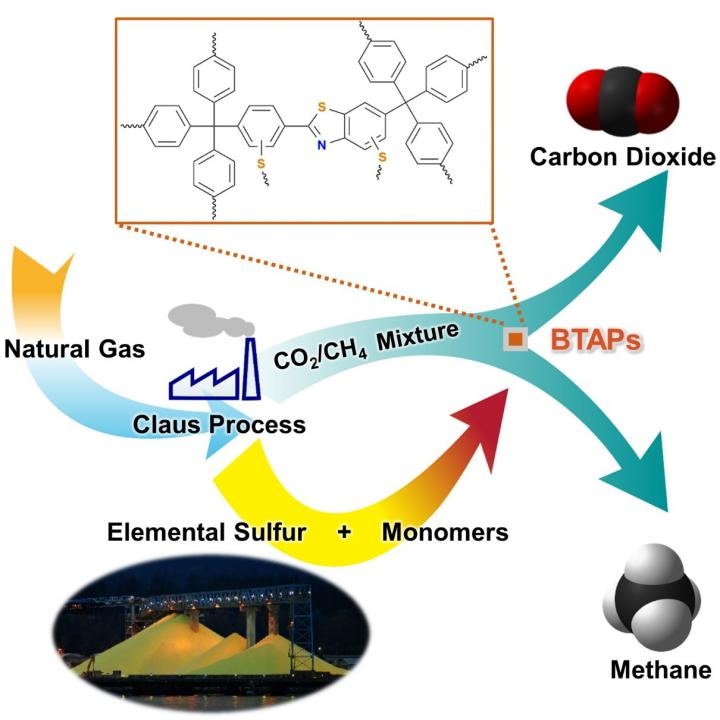
Methane, a key component of natural gas, has recently emerged as a vital source of energy due to its availability in huge quantities and its relatively clean nature compared to fossil fuels. However, natural gas can only be used as a fuel if it goes through a process called "natural gas sweetening" or "hydrodesulfurization" in order to reduce the emissions of sulfur-dioxide from combustion of fossil fuels.
This process results in involuntary and excessive production of elemental sulfur. Even though sulfur is one of the globally established common and versatile elements, it has relatively few large-scale applications, mostly for sulfuric acid and gunpowder production.
There is still a challenge in the development of processing and synthetic methods that help converting sulfur into useful chemicals. Recently, a research team headed by Professor Ali Coskun from the Graduate School of EEWS (Energy, Environment, Water and Sustainability) at Korea Advanced Institute of Science and Technology (KAIST) launched a new method to solve this issue by directly using elemental sulfur in the synthesis of microporous polymers for the natural-gas sweetening process.
Natural gas, comprising of differing amounts of hydrogen sulfide (H2S) and carbon dioxide (CO2), is usually treated with amine solutions, and this is followed by regeneration of these solutions at increased temperatures in order to discharge captured H2S and CO2.
These gases are removed with the help of a two-step separation process. First, the amine solutions remove H2S, and then separation of CO2 from methane (CH4) takes place with either amine solutions or porous sorbents, such as microporous polymers.
The researchers used elemental sulfur and organic linkers and created a solvent and catalyst-free strategy to carry out the synthesis of ultramicroporous benzothiazole polymers (BTAPs) in quantitative yields. BTAPs were identified as being very porous and exhibited excellent physiochemical stability.
The CO2 affinity of the sorbent was increased by the in-situ chemical impregnation of sulfur within the micropores, and the diffusion of CH4 was limited. BTAPs, as cost-effective, scalable solid-sorbents, displayed excellent CO2 separation ability for flue gas, and also for natural and landfill gas conditions.
The team noted that: "Each year, millions of tons of elemental sulfur are generated as a by-product of petroleum refining and natural-gas processing, but industries and businesses lacked good ideas for using it. Our research provides a solution: the direct utilization of elemental sulfur into the synthesis of ultramicroporous polymers that can be recycled back into an efficient and sustainable process for CO2 separation. Our novel polymeric materials offer new possibilities for the application of a little-used natural resource, sulfur, to provide a sustainable solution to challenging environmental issues."