May 15 2017
With the help of an atomic force microscope, researchers from the University of Basel's Swiss Nanoscience Institute network have managed to study the strength of hydrogen bonds in a single molecule for the first time. They have reported their findings in the Science Advances journal.
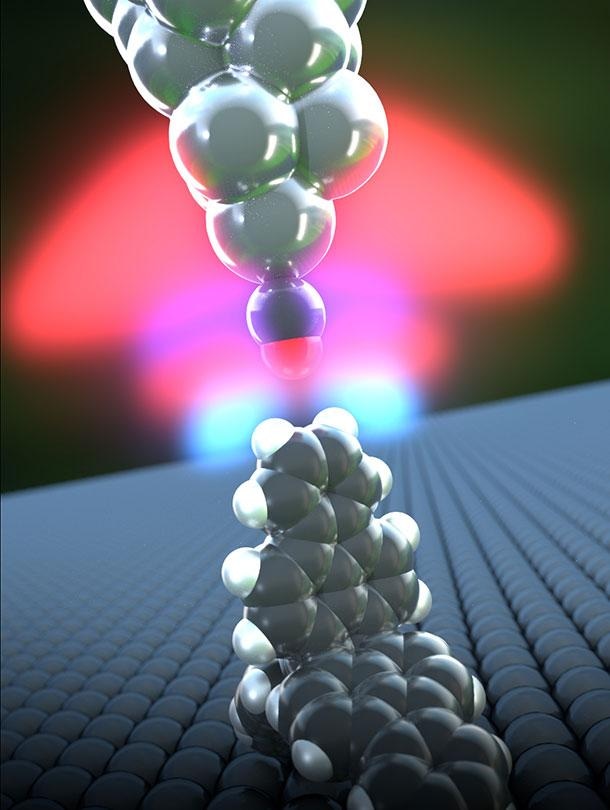 A hydrogen bond forms between a propellane (lower molecule) and the carbon monoxide functionalized tip of an atomic force microscope. The measured forces and the distance between the oxygen atom at the AFM tip and the propellane's hydrogen atoms correspond precisely to the calculations. (Credit - University of Basel, Department of Physics)
A hydrogen bond forms between a propellane (lower molecule) and the carbon monoxide functionalized tip of an atomic force microscope. The measured forces and the distance between the oxygen atom at the AFM tip and the propellane's hydrogen atoms correspond precisely to the calculations. (Credit - University of Basel, Department of Physics)
The most common element in the universe is hydrogen and it forms an integral part of nearly all organic compounds. Molecules and sections of macromolecules are linked to each other via hydrogen atoms, an interaction termed as hydrogen bonding. These interactions have a crucial role to play in nature, because they are accountable for specific properties of proteins or nucleic acids and, for instance, also guarantee that water has a high boiling temperature.
So far, it has not been possible to do an electron microscopic or spectroscopic analysis of hydrogen and the hydrogen bonds in single molecules, and studies using atomic force microscopy have also not produced any accurate results.
Dr. Shigeki Kawai, from Professor Ernst Meyer's team at the Swiss Nanoscience Institute and the Department of Physics at the University of Basel, has currently used a high-resolution atomic force microscope to successfully investigate hydrogen atoms in individual cyclic hydrocarbon compounds.
Choosing the right molecules for a clear view
In close partnership with colleagues from Japan, the researchers chose compounds whose configuration look like that of a propeller. These propellanes organize themselves on a surface in such a way that two hydrogen atoms constantly point upwards. If the tip of the atomic force microscope, which is functionalized with carbon monoxide, is taken sufficiently close to these hydrogen atoms, hydrogen bonds are created that can then be studied.
Hydrogen bonds are a lot weaker than chemical bonds, but stronger than intermolecular van der Waals interactions. The measured forces and distances between the oxygen atoms at the tip of the atomic force microscope and the propellane's hydrogen atoms match superbly to the calculations performed by Prof. Adam S. Foster from Aalto University in Finland. They display that the interaction obviously involves hydrogen bonds. The measurements mean that the much weaker van der Waals forces and the stronger ionic bonds can be omitted.
With this research, the team from the University of Basel's Swiss Nanoscience Institute network has paved the way to identify 3D molecules such as polymers or nucleic acids via observation of hydrogen atoms.