Jul 27 2018
Airy, Airier, Aerogel. Aerogels are delicate solids, which contain almost entirely air-filled pores. Until now, the practical application of aerogels has been limited by brittleness. However, this may now change.
 © Wiley-VCH
© Wiley-VCH
In the journal Angewandte Chemie, Japanese scientists have now developed highly elastic aerogels that can be easily processed and can also be produced cost-effectively. Their success depends on a doubly cross-linked organic-inorganic network structure with modifiable network density.
Aerogels can be composed of a wide range of materials; however, these materials are brittle making it almost impossible to perform drilling, cutting, or milling processes. Moreover, the drying process required by aerogels is generally very costly.
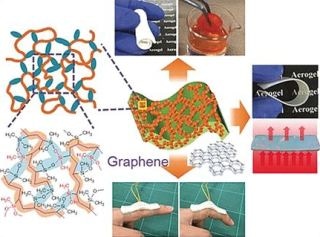
In the research team, headed by Kazuyoshi Kanamori and Kazuki Nakanishi, at Kyoto University (Japan), Guoqing Zu has now created a novel class of extraordinarily elastic aerogels. The team’s materials are based on vinylmethyldimethoxysilane and vinyldimethylmethoxysilane - monomers that are initially connected into polymer chains by a radical polymerization through the double bonds in their vinyl groups. Within the polymer chains, there are organosilicon side chains (silanes), which – based on the monomer – possess one or two methoxy groups (–OCH3) on the silicon atom.
These groups take part in the subsequent step - a cross-linking reaction at one or two sites in the silane side chain, again based on the monomer. Eventually, siloxane bridges (–Si–O–Si–) are formed by this polycondensation. The density of the ensuing cross-linking of the polymers (polyvinylpolydimethylsiloxane-polyvinylpolymethylsiloxane copolymers) relies on the ratio wherein both monomers were combined together. Subsequent low-cost freeze drying or air pressure forms aerogels with customized porosity.
The delicate structures, with their flexible hydrocarbon and siloxane chains, are extremely elastic. These structures can be twisted, rolled, bent, and cut into preferred shapes. Crosslinked versions, which are denser, have high thermal insulation, exceeding conventional materials like polyurethane foam.
The selective absorption of the aerogels is also of interest. When aerogels are exposed to a hexane-water mixture, they absorb the hexane, which can then be removed by evaporation or by squeezing the material like a sponge. The process can be performed again until the complete separation of the mixture. This enables the separation of oils or solvents like toluene, acetone, kerosene, and mineral oil if they inadvertently enter a water body, for instance.
In addition, the team has produced composites using these polymers as well as electrically conducting graphene nanoplatelets. When the graphene platelets are under pressure, they move closer together and reversibly boost the conductivity. Touchpads for wearable electronics and electronic devices are potential uses for this property.