Feb 1 2019
When people exercise, the strain produced disintegrates fibers in the muscles. Human body constantly provides amino acids to repair those fibers, where the amino acids are mended into proteins, eventually developing stronger muscles.
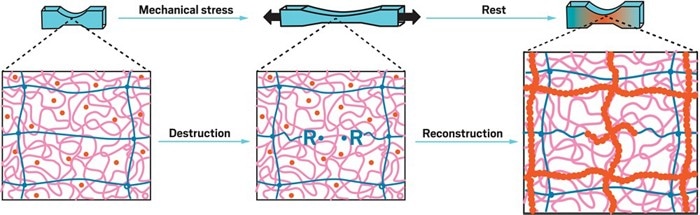 When the muscle-inspired hydrogel is stretched, its taut polymer (blue) breaks, forming radicals, and its slack polymer (pink) stretches to prevent damage to the hydrogel. The radicals react with monomer (orange) that is embedded in the polymer to form new polymer chains that strengthen the hydrogel. (Image credit: ACS)
When the muscle-inspired hydrogel is stretched, its taut polymer (blue) breaks, forming radicals, and its slack polymer (pink) stretches to prevent damage to the hydrogel. The radicals react with monomer (orange) that is embedded in the polymer to form new polymer chains that strengthen the hydrogel. (Image credit: ACS)
At present, researchers have made a muscle-like hydrogel that functions in a similar manner by strengthening itself when mechanically stressed. The proposed research could result in soft robots or longer-lasting tires composed of flexible plastic that can repair themselves and even grow.
A team headed by Hokkaido University’s Jian Ping Gong and Tasuku Nakajima developed the stretchy hydrogel material which leverages polymer mechanochemistry that involves the initiation of a chemical reaction by mechanical force. The soft but tough hydrogel is composed of 85% water and two intertwined, cross-linked polymer networks. One of the networks containing poly(2-acrylamido-2-methylpropanesulfonic acid) sodium salt is tight, whereas the other network containing polyacrylamide is saggy.
Upon pulling, the saggy polymer extends, which prevents the hydrogel from tearing, while on the other hand, the taut polymer breaks, creating carbon radicals at the broken ends of the polymer chains. These radicals rapidly react with monomer spread across the material to develop the polymer network again so that it becomes stronger than it was in the beginning when the hydrogel returns to its relaxed state. The scientists illustrate this by demonstrating that the material can lift heavier weights every time it is stretched.
Developing a molecular architecture that consists of adequate radicals to cause a macroscopic change in the hydrogel’s properties is a technical achievement and signifies “a significant step forward in the area of smart responsive polymers,” says Eindhoven University of Technology’s Rint P. Sijbesma, an expert in smart materials. According to him, this work is contrary to previous efforts where scientists attempted to create similar systems but were unsuccessful since the polymer architecture they used didn’t produce adequate radicals to make a visible difference in strength.
Nakajima mentions that nevertheless there are numerous possibilities for improvement in the system. For instance, he states, the scientists must find out a way to constantly supply monomer to the system. In the existing system, the monomer gets used up following five to six stretches, and the hydrogel becomes rigid and brittle. In addition, the system is sensitive to oxygen, a common issue in radical polymerizations.
Conceptually the advance is inspiring. While chemists are comfortable performing chemistry in the controlled environment of a flask, this work pushes us to think about the challenges of chemistry in the wild, robust enough to work under demanding conditions.
Jeffrey S. Moore, Polymer Mechanochemistry Expert, University of Illinois.