Mar 25 2019
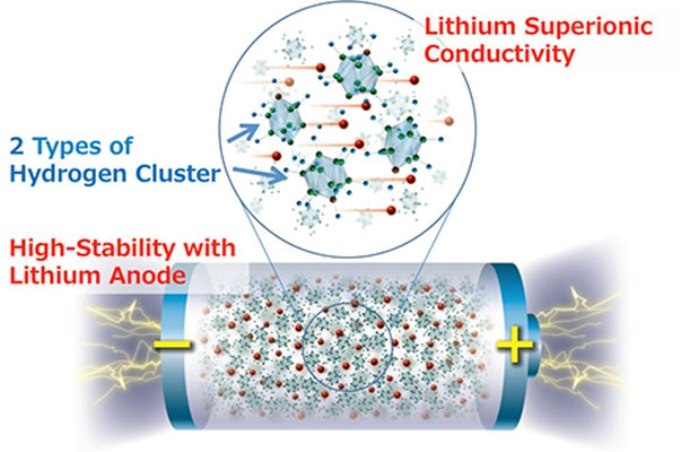
Researchers from Tohoku University and the High Energy Accelerator Research Organization have created a new complex hydride lithium superionic conductor that could yield all-solid-state batteries with the highest energy density so far.
 High-energy-density all-solid-state lithium metal battery employing complex hydrides. (Image credit: Sangryun Kim and Shin-ichi Orimo)
High-energy-density all-solid-state lithium metal battery employing complex hydrides. (Image credit: Sangryun Kim and Shin-ichi Orimo)
The scientists state that the new material, obtained by developing structures of hydrogen clusters (complex anions), exhibits distinctly high stability against lithium metal, which would make it the supreme anode material for all-solid-state batteries.
All-solid-state batteries containing a lithium metal anode have the capability to tackle the energy density problems of traditional lithium-ion batteries. However, so far, their use in practical cells has been restricted by the high lithium ion transfer resistance, chiefly due to the instability of the solid electrolyte against lithium metal.
This new solid electrolyte that shows high stability and high ionic conductivity against lithium metal can thus be actual progress for all-solid-state batteries using a lithium metal anode.
We expect that this development will not only inspire future efforts to find lithium superionic conductors based on complex hydrides but also open up a new trend in the field of solid electrolyte materials that may lead to the development of high-energy-density electrochemical devices.
Sangryun Kim, Tohoku University.
Sangryun Kim belongs to Shin-ichi Orimo’s research group.
Background
All-solid-state batteries are potential candidates for overcoming the inherent disadvantages of existing lithium-ion batteries, like flammability, electrolyte leakage, and restricted energy density.
Lithium metal is generally assumed to be the best anode material for all-solid-state batteries as it has the highest theoretical capacity (3860 mAh g−1) and the lowest potential (−3.04 V vs standard hydrogen electrode) among established anode materials.
Lithium-ion-conducting solid electrolytes are an important element of all-solid-state batteries since the battery performance is decided by the ionic conductivity and stability of the solid electrolyte.
The challenge is that a majority of the existing solid electrolytes have chemical/electrochemical instability and/or poor physical contact against lithium metal, unavoidably leading to unnecessary side reactions at the interface. These side reactions lead to an increase in interfacial resistance, significantly deteriorating battery performance during repeated cycles.
As demonstrated by earlier studies, which presented approaches such as alloying the lithium metal and interface modification, this degradation process is extremely complicated to deal with as its origin is the high thermodynamic reactivity of the lithium metal anode with the electrolyte.
High stability and high lithium ion conductivity of the solid electrolyte are the major challenges to using the lithium metal anode.
Complex hydrides have received a lot of attention in addressing the problems associated with the lithium metal anode because of their outstanding chemical and electrochemical stability against the lithium metal anode. But because of their low ionic conductivity, using complex hydrides with the lithium metal anode have never been attempted in practical batteries. So we were very motivated to see if developing complex hydride that exhibits lithium superionic conductivity at room temperature can enable the use of lithium metal anode. And it worked.
Sangryun Kim, Tohoku University.
The Tohoku University research group was headed by Sangryun Kim from the Institute of Material Research (IMR) and Shin-ichi Orimo from the Advanced Institute for Materials Research (AIMR). Members included Dorai Arunkumar, Naoaki Kuwata and Junichi Kawamura from the university’s Institute of Multidisciplinary Research for Advanced Materials (IMRAM), and also Toshiya Otomo from the High Energy Accelerator Research Organization.