Apr 17 2019
Designing a lithium-ion battery that can charge within minutes yet work at a high capacity is possible, according to a research paper from Rensselaer Polytechnic Institute (RPI) recently published in Nature Communications. This development shows the potential to enhance battery performance for solar grid storage, consumer electronics, and electric vehicles.
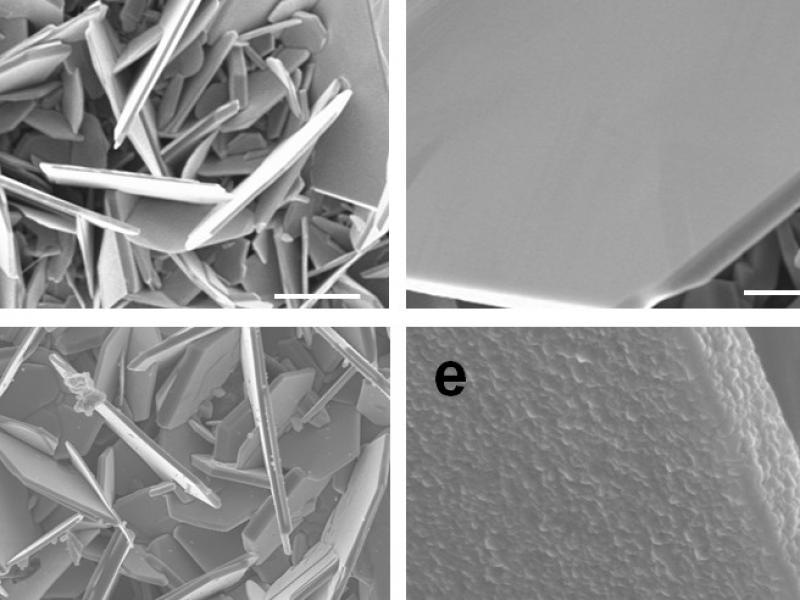 VS2 flakes with and without TiS2 coating. (Image credit: Rensselaer Polytechnic Institute)
VS2 flakes with and without TiS2 coating. (Image credit: Rensselaer Polytechnic Institute)
A lithium-ion battery charges and discharges as lithium ions travel between two electrodes, called a cathode and an anode. In a traditional lithium-ion battery, the anode is composed of graphite, while the cathode is made of lithium cobalt oxide.
These materials work well together, which is why lithium-ion batteries have become progressively widespread, but scientists at RPI believe the function can be improved further.
“The way to make batteries better is to improve the materials used for the electrodes,” said Nikhil Koratkar, professor of mechanical, aerospace, and nuclear engineering at RPI, and corresponding author of the paper. “What we are trying to do is make lithium-ion technology even better in performance.”
Koratkar’s wide-ranging research into nanotechnology and energy storage has positioned him among the world’s most highly cited scientists. In this latest work, Koratkar and his team enhanced performance by switching cobalt oxide with vanadium disulfide (VS2).
It gives you higher energy density, because it’s light. And it gives you faster charging capability, because it’s highly conductive. From those points of view, we were attracted to this material.
Nikhil Koratkar, Professor of Mechanical, Aerospace, and Nuclear Engineering, Department of Materials Science and Engineering, RPI.
There has been a lot of excitement regarding the potential of VS2 in recent years, but so far, Koratkar said, scientists had been defied by its instability—a feature that would result in short battery life. The RPI scientists not only demonstrated why that instability was occurring, but also developed a way to beat it.
The interdisciplinary team, which also included Vincent Meunier, head of the Department of Physics, Applied Physics, and Astronomy, and others, established that lithium insertion caused an irregularity in the spacing between vanadium atoms, referred to as Peierls distortion, which was liable for the disintegration of the VS2 flakes. They learned that covering the flakes with a nanolayered coating of titanium disulfide (TiS2)—a material that does not Peierls distort—would steady the VS2 flakes and enhance their performance within the battery.
This was new. People hadn’t realized this was the underlying cause. The TiS2 coating acts as a buffer layer. It holds the VS2 material together, providing mechanical support.
Nikhil Koratkar, Professor of Mechanical, Aerospace, and Nuclear Engineering, Department of Materials Science and Engineering, RPI.
Once that issue was solved, the team discovered that the VS2-TiS2 electrodes could work at a high specific capacity, or store plenty of charge per unit mass. Koratkar stated that vanadium and sulfur’s small size and weight permit them to supply a high energy density and capacity. Their small size would also benefit the battery’s compactness.
When charging took place more rapidly, Koratkar said, the capacity did not drop as considerably as it frequently does with other electrodes. The electrodes were able to sustain a practical capacity because, in contrast to cobalt oxide, the VS2-TiS2 material is electrically conductive.
Koratkar sees numerous applications for this discovery in enhancing car batteries, power for portable electronics, and solar energy storage where high capacity is crucial, but better-charging speed would also be appealing.