Jul 22 2019
Scientists in Japan have developed simulations that could offer new perceptions about the reactions taking place in solid-oxide fuel cells by using practical atomic-scale models of the active site at the electrode based on microscope observations as the preliminary point.
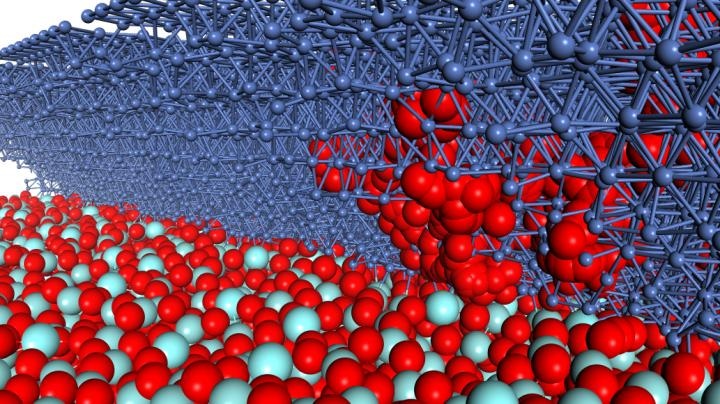 The initial positions of the atoms in this computer model of a solid-oxide fuel cell were based on observations of the actual atomic configuration using electron microscopy. Simulations using this model revealed a previously unreported reaction (red path) in which an oxygen molecule from the yttria-stabilized zirconia layer (layer of red and light blue balls) moves through the bulk nickel layer (dark blue balls) before forming OH on the nickel surface. (Image credit: Michihisa Koyama, Kyushu University)
The initial positions of the atoms in this computer model of a solid-oxide fuel cell were based on observations of the actual atomic configuration using electron microscopy. Simulations using this model revealed a previously unreported reaction (red path) in which an oxygen molecule from the yttria-stabilized zirconia layer (layer of red and light blue balls) moves through the bulk nickel layer (dark blue balls) before forming OH on the nickel surface. (Image credit: Michihisa Koyama, Kyushu University)
This better insight could offer hints on ways to enhance the durability and performance in future devices. Highly promising for the generation of clean and efficient electricity, solid-oxide fuels cells yield electricity via the electrochemical reaction of a fuel with air. They have already started to find their way into homes and office buildings across Japan.
In a standard fuel cell, oxygen molecules on one side of the fuel cell first receive electrons and disintegrate into oxide ions. Then, the oxide ions move through an electrolyte to the other side of the device, where they react with the fuel and discharge their additional electrons. These electrons travel through outer wires back to the beginning side, finishing the circuit and powering anything that is connected to the wires.
Although this general reaction is widely known and comparatively simple, the reaction step restricting the overall rate of the process remains controversial as the complex structures of the electrodes—which are usually porous materials as opposed to basic, flat surfaces—hamper the investigation of the occurrences at the atomic level.
Since comprehensive knowledge about the reactions taking place in the devices is essential for additionally improving the durability and performance of fuel cells, the challenge has been to comprehend how the microscopic structures—down to the arrangement of the atoms at the various interfaces—influence the reactions.
Computer simulations have played a powerful role in predicting and understanding reactions that we cannot easily observe on the atomic or molecular scale. However, most studies have assumed simplified structures to reduce the computational cost, and these systems cannot reproduce the complex structures and behavior occurring in the real world.
Michihisa Koyama, Team Lead, INAMORI Frontier Research Center, Kyushu University
Koyama’s team focused on overcoming these inadequacies by applying simulations with improved parameters to realistic models of the main interfaces based on microscopic observations of the real positions of the atoms at the electrode’s active site.
Exploiting the strength of Kyushu University’s Ultramicroscopy Research Center, the scientists meticulously examined the atomic structure of thin slices of the fuel cells using atomic-resolution electron microscopy. Based on these examinations, the scientists then recreated computer models with the same atomic structures for two demonstrative arrangements that they examined.
Reactions between oxygen and hydrogen in these computer-generated fuel cells were then replicated with a technique called Reactive Force Field Molecular Dynamics, which involves applying a set of parameters to estimate how atoms will interact—and even chemically respond—with each other, without going into the total complexity of rigorous quantum chemical calculations.
Here, the scientists used an enhanced set of parameters formulated in partnership with Yoshitaka Umeno’s group at the University of Tokyo.
Studying the result of numerous runs of the simulations on the various model systems, the scientists learned that the preferred reactions were more likely to take place in the layers with smaller pore size.
Moreover, they found a new reaction pathway wherein oxygen travels through the bulk layers in a way that could possibly degrade durability and performance. Therefore, strategies to prevent this potential reaction route path should be considered as the scientists work to engineer better fuel cells.
These are the kinds of insights that we could only get by looking at real-world systems. In the future, I expect to see more people using real-world atomic structures recreated from microscope observations for the basis of simulations to understand phenomena that we cannot easily measure and observe in the laboratory.
Michihisa Koyama, Team Lead, INAMORI Frontier Research Center, Kyushu University