Nov 8 2019
A team of researchers from the National Institutes of Natural Sciences (ExCELLS/IMS) and Osaka University has unraveled the precise mechanism of the biosynthesis of carbon monoxide vital for the maturation of the active site of NiFe-hydrogenase.
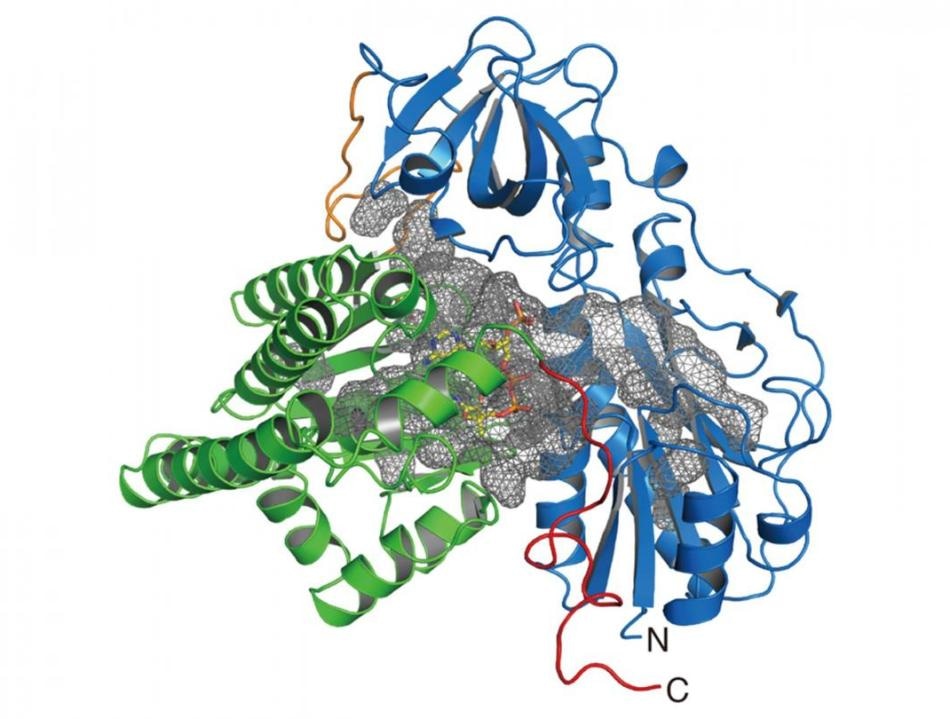 Crystal structure of HypX. The N- and C-terminal domains are shown in blue and green, respectively. The cavity inside of HypX is shown in a gray mesh. Image Credit: Institute for Molecular Science, National Institutes of Natural Sciences.
Crystal structure of HypX. The N- and C-terminal domains are shown in blue and green, respectively. The cavity inside of HypX is shown in a gray mesh. Image Credit: Institute for Molecular Science, National Institutes of Natural Sciences.
The study has been published in the October 18th issue of Communications Biology.
Background
Hydrogenase is a metalloenzyme that causes the oxidation of hydrogen gas and the reduction of proton. It plays a crucial role in bacterial hydrogen metabolism. Depending on the variances of metal content of the active site, they are divided into three groups: FeFe-, NiFe-, and Fe-hydrogenases comprising different metal complexes as active centers in these enzymes.
Despite the fact that the structures of the active centers of the enzymes differ, it is vital for hydrogenase activity that carbon monoxide (CO) is coordinated to the iron ion in the active center. Although CO is known to be biosynthesized by an enzymatic reaction, how this takes place was unidentified until now.
Result
In this study, the research team established the crystal structure of the enzyme (HypX) that causes the biosynthesis of CO, based on which HypX biosynthesizes CO by exceptional reaction for the maturation of NiFe-hydrogenase. HypX comprises two domains—the C-terminal and N-terminal domains.
A continuous cavity that links the N- and C-terminal domains exists in the inner side of HypX. The coenzyme A (CoA) in the crystal structure is attached in the C-terminal region of the cavity.
Two different reactions take place in the N- and C-terminal domains. In the N-terminal domain, formyl-group transfer reaction from formyltetrahydrofolate, attached in the N-terminal region of the cavity as a substrate, to CoA occurs (reaction step 1).
During this process, CoA in the cavity assumes the linear extended conformation, and the SH-group of CoA is positioned near the formyl group in the formyltetrahydrofolate attached in the N-terminal domain. Then, formyl-CoA forms as a reaction intermediate through the formyl-group transfer reaction from formyltetrahydrofolate to CoA.
In the following step, formyl-CoA experiences a large conformational change in the cavity so that the formyl group in the terminal position of formyl-CoA is situated at the active site in the C-terminal domain of HypX. In the C-terminal domain, CO is developed by decarbonylation of formyl-CoA.
This CO biosynthesis reaction is an exceptional and unique reaction. CoA is recognized as a coenzyme, which has a vital role in cellular energetic metabolism and fatty acid metabolism through the citric acid cycle. But CoA/formyl-CoA has never before been reported to be involved in CO biosynthesis reactions. This study has unraveled a novel physiological function of a well-established coenzyme CoA.
Future Prospects
The biosynthetic mechanisms of metalloenzymes remain unidentified in a number of cases. How the metal-containing active centers of metalloenzymes have been assembled is yet to be revealed.
In this research, the team established the first crystal structure of the enzyme that catalyzes the biosynthetic reaction of carbon monoxide vital to the construction of the active site of (NiFe) hydrogenase. Going forward, they will pursue the research for finding out the precise mechanism of the entire hydrogenase maturation pathway based on this outcome.