Nov 24 2020
At RUDN University, a chemist has proposed a basic and precise technique for producing the analogs of two natural toxins—septicine and antofine. This universal technique can even be applied to make other biologically active substances for medicinal chemistry.
 A chemist from RUDN University suggested a simple and accurate method for the synthesis of analogs of two natural toxins, antofine and septicine. This universal approach can also be used to obtain other biologically active substances for medicinal chemistry. Image Credit: RUDN University
A chemist from RUDN University suggested a simple and accurate method for the synthesis of analogs of two natural toxins, antofine and septicine. This universal approach can also be used to obtain other biologically active substances for medicinal chemistry. Image Credit: RUDN University
Details of the study have been published in the Organic Letters journal.
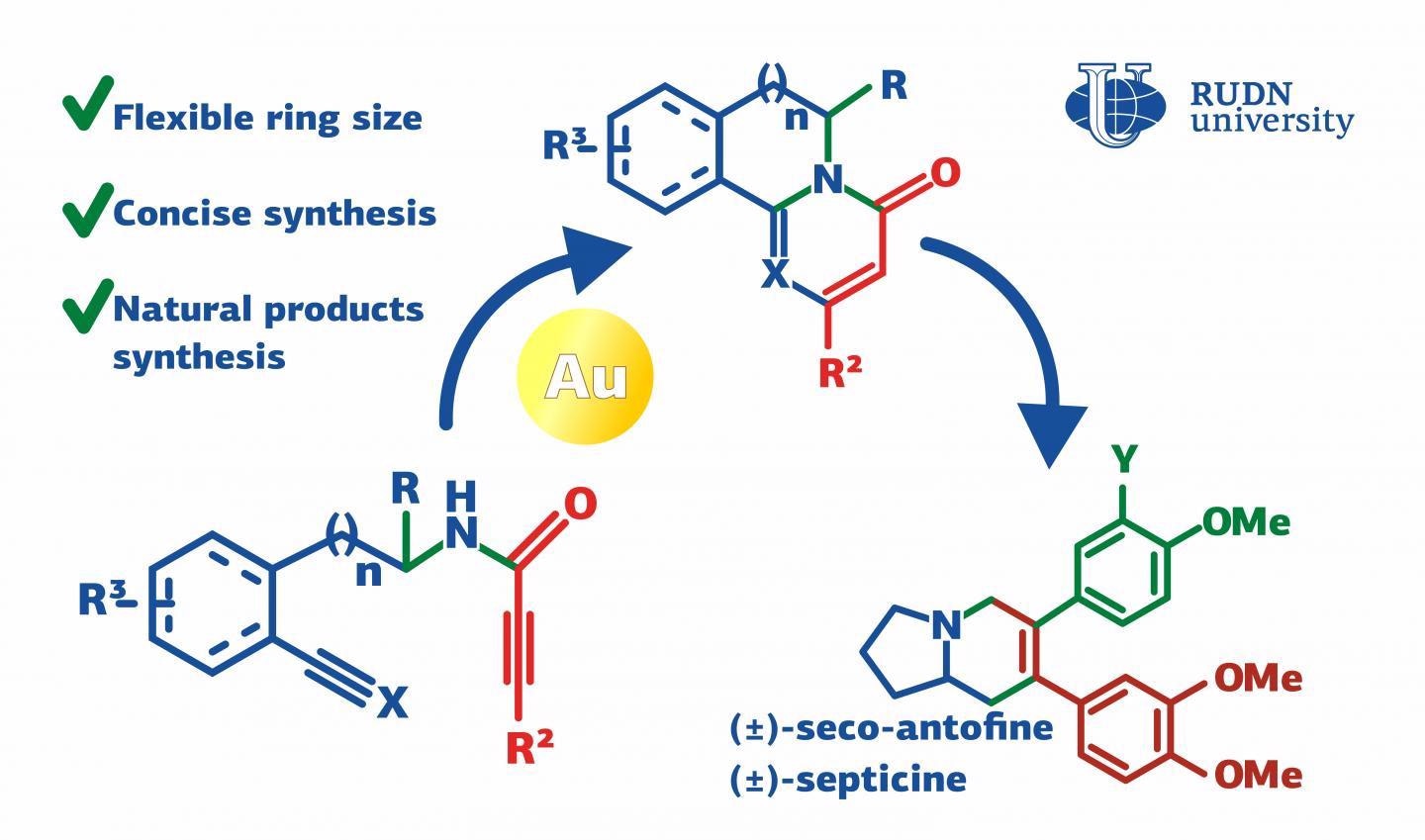
Septicine and antofine have antitumor and antibacterial properties and thus can be employed in the pharmaceutical sector. But it is hard to extract them straight from natural plant sources: they are hydrophobic and can occasionally be poisonous unpredictably.
The chemist has formulated a universal technique to synthesize 2-pyridone derivatives on which septicine and antofine are based. 4-pyrimidone and 2-pyridone are cyclic molecules with one oxygen atom bound to the cycle. The cycle of the previous compound has one nitrogen atom, and that of the latter has two. The two substances are used as molecular frameworks for medicinal drugs.
By connecting extra cycles and atoms to them, compounds with antiviral, antitumor, antimalarial, and anti-inflammatory properties can be obtained. The new technique of producing 2-pyridone and 4-pyrimidone includes just two steps.
The primary step is the so-called four-component Ugi reaction. Researchers can use this step to obtain peptide fragments (analogs of proteins) from four basic substances. In his experiment, the chemist used various substances at room temperature and the Ugi reaction took place for 12 hours.
Consequently, about 60 different compounds were achieved, and in all of them, a hydrocarbon ring was linked to other groups of atoms with peptide bonds.
In the following step, a number of new cycles had to be developed in the peptide fragment, and at least one of them had to have nitrogen atoms. To realize this, the chemist proposed using gold-based catalysts. Among the five catalysts that were tested, one delivered the best 2-pyridone yield (75%).
This result was realized in the reaction with the first of the manufactured peptide fragments. Additional studies revealed that the use of other first-stage products could result in up to 93% yield of the products with the needed molecular frameworks. The same method was applied to the production of 4-pyrimidone derivatives.
The main advantage of our method is the ability to develop organic substances based on heterocycles with different functional groups. This advantage appears at the stage of the four-component Ugi reaction, as many simple and affordable reagents can be used in it.
Erik Van der Eycken, Head, Joint Institute for Chemical Research, RUDN University
“The cyclization reaction suggested by us can involve reagents with different functional groups. The simplicity of this approach and a wide range of potential results make it favorable for medicinal chemistry,” concluded Van der Eycken.
Journal Reference:
Tian, G., et al. (2020) A Gold(I)-Catalyzed Hydroamination/Cycloisomerization Cascade: Concise Synthesis of (±)-seco-Antofine and (±)-Septicine. Organic Letters. doi.org/10.1021/acs.orglett.0c03062.