Jan 19 2021
Researchers have developed a new technique that makes the synthesis of acetals simpler and more eco-friendly. Acetals are key chemical compounds used, for instance, to produce specific medical agents.
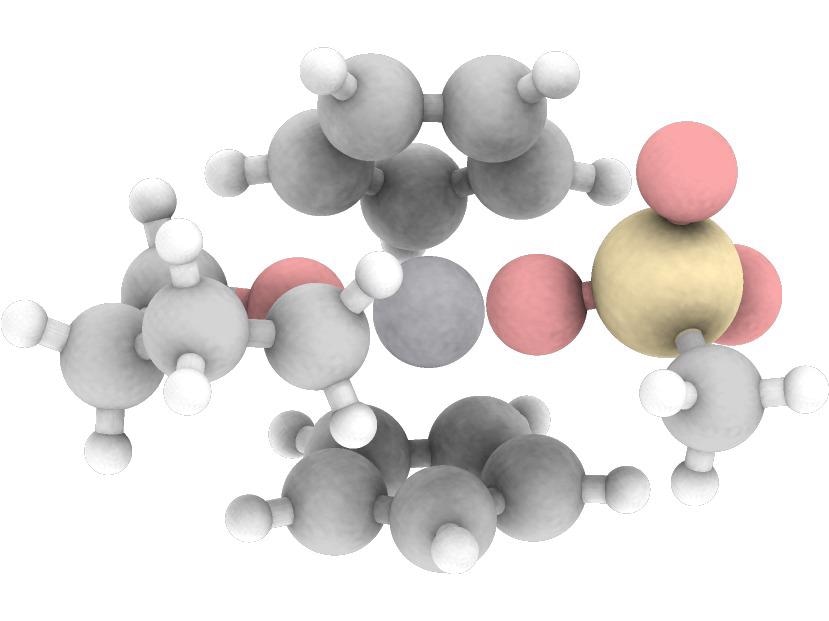 Computer-generated structure of the catalyst complex with the reduced titanium in the active center. Image Credit: © AG Prof. Grimme, Fabian Bohle/University of Bonn.
Computer-generated structure of the catalyst complex with the reduced titanium in the active center. Image Credit: © AG Prof. Grimme, Fabian Bohle/University of Bonn.
At the University of Bonn, chemists have devised and improved the new sustainable catalytic method. The researchers also used the most advanced computer simulations. The reaction is based on a mechanism that often takes place in nature, but has not been used much in chemical synthesis to date. The findings of the study have been reported in the Angewandte Chemie journal.
During acetal production, the main step is the bonding of two oxygen atoms to a single carbon atom. In general, chemists realize this arrangement through oxidation. Powerful oxidizing agents are often used to achieve this by discharging an oxygen atom during the reaction. It is essential to dispose of the remaining oxidizing agent after the synthesis.
In our study, however, we describe a path that is referred to as atomic-economic, meaning that it does not generate waste. The starting molecule itself already contains the oxygen atom required for oxidation. The catalysis we have developed simply shifts this oxygen in the molecule, which creates the acetal.
Dr Andreas Gansäuer, Professor, Kekulé Institute of Organic Chemistry and Biochemistry, University of Bonn
The starting molecule includes what is called an epoxy group, a type of “triangle” where two corners are formed by carbon atoms and the third one includes an oxygen atom. Triple rings such as these remain under great tension and thus easily disintegrate at the oxygen atom. Epoxies tend to store the required reaction energy similar to a compressed spring.
Catalysis Based on the Model of Nature
To achieve this goal, the use of an appropriate catalyst is required. In a metaphorical sense, oxygen atoms include two “arms” that can be used to form bonds. In case the epoxy ring disintegrates, one of the oxygen arms becomes free. Now, the catalyst binds to it temporarily.
This triggers a series of molecule-internal (intramolecular) rearrangements. When the series ends, the oxygen atom discharges the catalyst again and instead gets attached to the preferred carbon. “This step is called oxygen rebound,” noted Gansäuer.
This mechanism has not been used much in chemical syntheses until now—much contrary to nature: For instance, the liver uses the “oxygen rebound” to disintegrate toxins. This also necessitates catalysts, or what are called the P450 enzymes. Their active center includes an iron atom.
“The heart of our catalyst also consists of a common and non-toxic metal, namely titanium,” explained Prof. Dr Stefan Grimme from the Institute of Physical and Theoretical Chemistry at the University of Bonn.
Catalyst Tuning on the Computer
When acetal synthesis occurs, an oxygen atom is first absorbed by titanium, which then releases it again (oxidation follows what is called reduction). This is effective only if it binds the oxygen sufficiently strong to itself without “clinging” much. Its oxygen affinity is suitably adjusted by binding titanium to specific molecules, that is, its ligands.
Based on the binding partner, the metal has a marginally stronger oxidizing effect or can be reduced more easily. A computer is used these days to select the most appropriate “tuning molecules.” The research team led by Prof. Grimme specializes in this task: In the past few years, the team has created algorithms that enable extremely fast simulations of catalyst properties.
This allowed the researchers of this study to improve their catalyst such that it entirely converts the starting material into the preferred acetal.
The result documents very nicely how useful close cooperation between experiment and theory is for developing sustainable catalysis methods.
Dr Andreas Gansäuer, Professor, Kekulé Institute of Organic Chemistry and Biochemistry, University of Bonn
Dr. Gansäuer and Dr. Grimme are both members of the transdisciplinary research area (TRA) “Building blocks of matter and fundamental interactions” at the University of Bonn. Six different TRAs bring together scientists from a broad range of faculties and disciplines to collaborate on future-relevant research topics of the University of Excellence.
The research was funded by the German Research Foundation DFG, with grants from the Gottfried Wilhelm Leibniz Prize and the Konrad Adenauer Foundation.
Journal Reference:
Funk, P., et al. (2021) Oxidation Under Reductive Conditions: From Benzylic Ethers to Acetals with Perfect Atom‐Economy by Titanocene(III) Catalysis. Angewandte Chemie. doi.org/10.1002/anie.202013561.