Lithium-ion batteries (LIBs) have shown considerable development with advances in technology and have revolutionized the world, powering everything right from smartphones to electric cars.
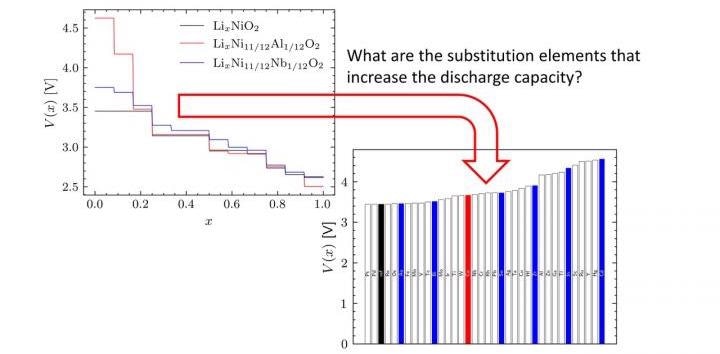 Scientists used density functional theory (DFT) and SCAN exchange-correlation functional to quantitatively derive reliable discharge profiles (right), alleviating the quantitative inconsistencies of traditional DFT. Image Credit: Ryo Maezono from JAIST.
Scientists used density functional theory (DFT) and SCAN exchange-correlation functional to quantitatively derive reliable discharge profiles (right), alleviating the quantitative inconsistencies of traditional DFT. Image Credit: Ryo Maezono from JAIST.
The next course in advancing the technology is to develop more optimized batteries that can power electronic devices for extended durations. One useful method for increasing the performance of the battery is the atomic substitution of positively charged ions or “cations” in the cathode material.
It is costly and complicated to perform this process in a systematic manner for various substituent cations to experimentally find the perfect ones, which leaves simulations as the only feasible option for identifying the choices.
Numerous studies have reported increased battery life and thermal stability depending on their findings with the help of a simulation-based method. Yet, these improvements reduced the battery’s discharge capacity, which is the amount of energy supplied by a battery in a single discharge. Consequently, a thorough search must be executed to find the cation substituent that improves the discharge capacity.
To this end, a research group headed by Professor Ryo Maezono from Japan Advanced Institute of Science and Technology (JAIST) has performed a broadscale screening of various cations for partial substitution of nickel in a nickel-based LIB to improve the discharge capacity of the battery.
The discharge capacity can be determined using the discharge profile, which is the voltage change during the charge-discharge process. We used first-principles calculations to evaluate the discharge profiles of materials that, in turn, determines their discharge capacities.
Ryo Maezono, Professor, Japan Advanced Institute of Science and Technology
“However, these calculations are computationally costly, so we integrated other methods to narrow down the candidates for cation replacement. To the best of our knowledge, this is the first study that successfully predicts cation substitution to increase battery capacity,” added Maezono.
The innovative study was published in the latest issue of The Journal of Physical Chemistry C.
The “strongly constrained and appropriately normed” (SCAN) functional is a notable approach for estimating the discharge voltage profile of a battery. However, these techniques seem to be impractical for extensive screening due to the huge computing charges.
Therefore, the researchers started the process by making use of affordable methods like cluster expansion and density functional theory to determine appropriate candidates for cation replacement. They applied SCAN functional to the inferred candidates to guarantee precision and reliability in voltage predictions.
The screening process showed that the highest discharge capacity was achieved when nickel was substituted partially with palladium and platinum in nickel-based LIB. These findings were correlative with the experimental data, thereby verifying the suggested methodology.
Professor Maezono stressed the need for additional studies and is confident about the future of their affordable screening process.
Our findings indicate that substituents such as rhenium and osmium offer high discharge capacities. However, these elements are rare and costly, and putting them to practical use would be challenging. Further study is needed to achieve the same effect with less substitution, multiple element substitution, or anion substitution.
Ryo Maezono, Professor, Japan Advanced Institute of Science and Technology
“Having said that, our novel computational technique will accelerate the search for optimal materials that improve battery performance at lower costs, allowing us to replace the bulk of our current electricity sources with carbon-free alternatives,” added Maezono.
The researchers hope that such progress will bring mankind a step forward to becoming an eco-friendly species in the years to come.
Journal Reference:
Yoshio, S., et al. (2021) High-Throughput Evaluation of Discharge Profiles of Nickel Substitution in LiNiO2 by Ab Initio Calculations. The Journal of Physical Chemistry C. doi.org/10.1021/acs.jpcc.0c11589.