At the North Carolina State University, scientists have come up with an affordable and fast method for generating hindered amines. Hindered amines are a class of chemicals utilized as building blocks in products varying from pharmaceuticals and agrochemicals to organic light-emitting diodes and detergents.
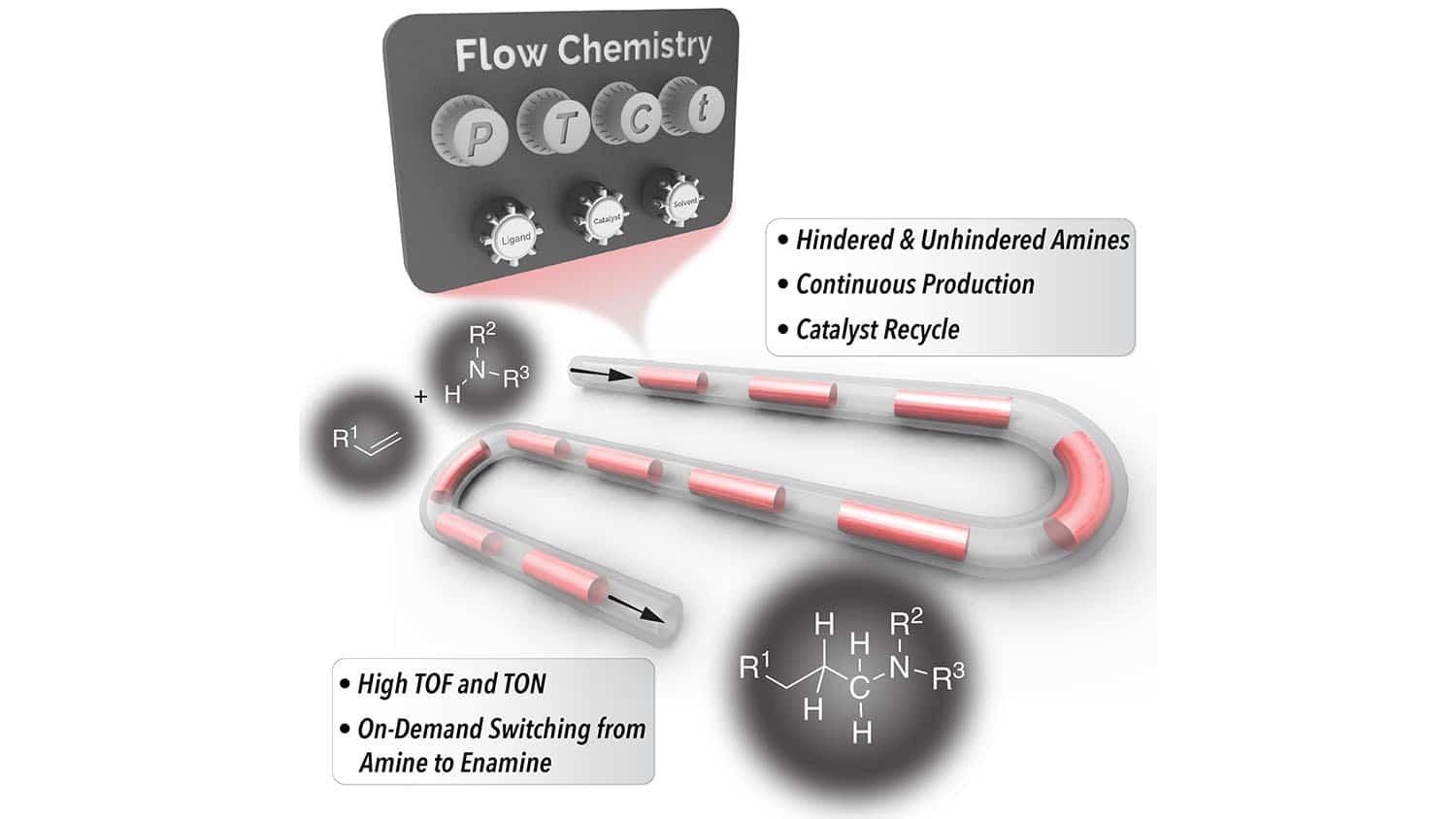
Image Credit: North Carolina State University.
Hindered amines are used in a tremendous variety of products, but all of the existing techniques for producing these amines are complicated and expensive. We set out to develop a better method for synthesizing these hindered amines, and we were successful.
Milad Abolhasani, Study Corresponding Author and Associate Professor, Chemical and Biomolecular Engineering, North Carolina State University
One of the affordable methods utilized for producing hindered amines is so-called hydroaminomethylation or HAM. But the chemical industry has largely avoided it since there are several things that can go wrong — thereby leaving producers with unwanted chemicals rather than the functionalized amines that they were trying to create.
For the past few years, scientists have enhanced the HAM process. However, all of the methods for preventing unwanted byproducts have meant expanding the timeframe of the HAM process. Hence, it takes hours to execute all of the essential reactions so far.
We’ve developed a HAM technique that makes use of continuous flow reactor technologies to produce hindered amines more efficiently. Our HAM process takes less than 30 minutes in most cases. The only products are hindered amines and water. And we are able to recycle the primary catalyst, rhodium/N-Xantphos, which further drives down costs.
Milad Abolhasani, Study Corresponding Author and Associate Professor, Chemical and Biomolecular Engineering, North Carolina State University
The success of the new method is made possible with the help of two things. Initially, by utilizing a constant flow reactor that enables the continuous flow of both gases and liquids in a segmented flow format, it was possible for scientists to make the kinetics of the reaction highly effective.
Second, the new method utilizes a co-catalyst — fluorinated benzoic acid — which decreases the amount of energy required to execute a few of the essential reactions in the HAM process.
Eventually, this method drives down the cost of producing impeded amines with the help of affordable feedstock, thereby enabling users to generate them in faster and with no harmful byproducts.
By designing a cooperative catalyst system, we’ve demonstrated that the rate of the HAM reactions in our system can be 70 times higher than the existing state-of-the-art processes.
Malek Ibrahim, Study First Author and Former Postdoctoral Researcher, North Carolina State University
Ibrahim added, “This process is also a good example for how flow chemistry platforms can improve catalyst turnover frequency, which is increasingly important as the price of rhodium catalysts goes up.”
The new method is particularly appealing for decentralized manufacturing operations since the compact footprint of the essential equipment and its scalability enables users to effectively produce hindered amines on-site and on-demand.
Ibrahim stated, “What’s more, the same technique can also be used to produce enamines—which are other chemical building blocks—on demand, simply by tuning the solvents we use in the flow reactor.”
Ibrahim adds, “You can literally switch back and forth between producing amines and enamines without having to stop the production process since the only thing you’re changing is the solvent mixture.”
A provisional patent has been filed by the scientists on the new method and the team is presently looking for industrial collaborators to put the method into extensive use.
Journal Reference:
Ibrahim, M Y S & Abolhasani, M (2022) Recyclable cooperative catalyst for accelerated hydroaminomethylation of hindered amines in a continuous segmented flow reactor. Nature Communications. doi.org/10.1038/s41467-022-30175-0.