A team of researchers recently published a paper in the journal Materials that reviewed the synthesis of nanostructured carbon (NC) from renewable resources, specifically from agricultural waste materials.

Study: Nano‐Structured Carbon: Its Synthesis from Renewable Agricultural Sources and Important Applications. Image Credit: Matauw/Shutterstock.com
Background
NC materials, such as fullerenes, carbon nanotubes (CNTs), graphene oxide, carbon nanoparticles (NPs), and carbon nanodots, have gained prominence for applications in environmental, biotechnology, energy, and biomedical fields owing to their high surface area and excellent thermal, electrical, mechanical, and chemical properties. NC materials can be synthesized using several strategies, including chemical vapor deposition, microwave methods, and pyrolysis.
The use of cheaper and renewable reactants and agricultural feedstocks is increasing for NC production to simplify the production process and reduce the cost of production. In this study, researchers reviewed the synthesis of NC using renewable sources. Specifically, the review focused on NC synthesis from agricultural waste materials using different methods and important applications of NCs.
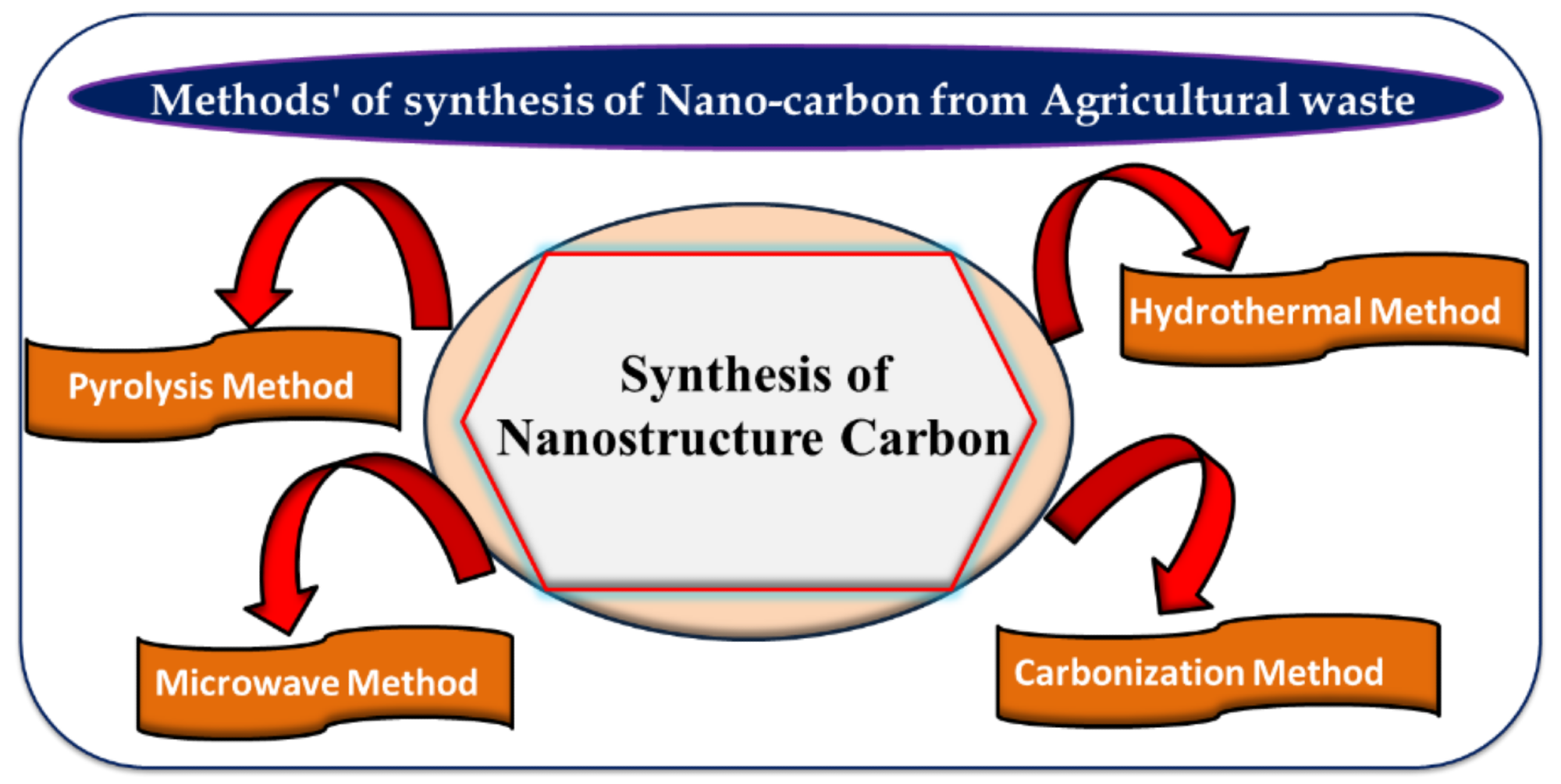
Various methods such as pyrolysis, microwave, hydrothermal, and carbonization for nanocarbon synthesis from agricultural waste. Image Credit: Singh, J et al., Materials
Methods Used for NC Synthesis from Agricultural Waste
Hydrothermal Method
The hydrothermal (HT) method is used to convert agricultural waste materials to carbon nanomaterials and fuels. HT processes are typically performed at high pressure in the presence of water. This method is eco-friendlier and more economical for NC synthesis as water as a solvent produces water vapor upon heating to generate a high pressure within a closed chamber.
Agro-wastes, such as banana peels, were dried and ground to homogenous fine particles and then placed in an HT reactor for six hours at 80 °C. The as-prepared product was washed repeatedly and collected by centrifugation. Eventually, the collected samples were dried to obtain banana-peel carbon.
Similarly, grape seeds, the waste products generated by the viniculture industry, were used to produce oil, and the residue left after oil production was used to produce NC materials. The physicochemical properties of synthesized carbon can be tuned by varying the substrate concentration, catalyst, and temperature in the HT process.
Microwave Hydro‐Thermal Carbonization (MHC) and General Carbonization
In MHC, microwave energy is utilized to heat the HT unit where the carbonization takes place. MHC method is faster compared to the normal HT method as microwave heating is a fast, economical, and efficient way to induce the carbonization reaction. Rice straw was initially chopped and ground to obtain homogeneously-sized straw dust and then placed in digestion tubes along with water.
Subsequently, the digestion units were placed in the microwave, and the temperature in the microwave was maintained at 230 °C to produce hydrochar with various physicochemical properties. These depended on parameters such as reaction time and temperature. The hydrochar can also be synthesized by placing a powdered-rice husk in the HT unit under microwave irradiation.
In general carbonization, agricultural waste carbonization was achieved using simply closed containers or by chemical pre-treatment in an open fire. Corn cabs, wheat straw, and rice husk were dried and ground into powders. The powders were chemically activated by adding sodium chloride in a fixed ratio.
Subsequently, the mixture was kept in an open wood fire for half an hour for carbonization in a low oxygen environment. In the Elsa barrel technique, biochar is produced from agricultural waste such as cassava, groundnut husk corn cobs, sawdust, and rice husk. Moreover, graphene oxide was also obtained from sugarcane bagasse.
![SEM image of SCBAC with 70×, 200× (left), and 1500× (right). Adapted with permission from Ref. [92].](https://d12oja0ew7x0i8.cloudfront.net/images/news/ImageForNews_59256_16545066616416496.png)
SEM image of SCBAC with 70×, 200× (left), and 1500× (right). Image Credit: Singh, J et al., Materials
Microwave Irradiation
The microwave irradiation technique is a crucial NC synthesis method due to its low energy consumption at various energy modes at high temperatures. The method primarily uses irradiation frequency to precisely control the duration and temperature of the synthesis process. Various carbon nanostructures with controlled shapes and porosity were obtained through this method.
For instance, micro cellulose derived from agro-waste products was utilized to synthesize microporous carbon sponges using microwave irradiation. Similarly, a simple microwave irradiation technique was used to produce biochar from waste palm oil kernels, while the microwave pyrolysis method was employed to synthesize a fluorescent carbon dot from natural sesame seeds.
Pyrolysis
Pyrolysis is a simple thermochemical method commonly used to disintegrate carbon sources into smaller components under anaerobic conditions. Although pyrolysis is traditionally utilized for petroleum product fractionation, this method is now gaining attention for recycling agricultural waste products into environmentally and economically beneficial products, such as NCs.
In pyrolysis, stalks of agricultural waste or wood were initially ground to decrease the particle size. Purely ground quartz silica in form of a small diameter tube of 60 cm length was used in the tubular furnace. A vacuum chamber was utilized to create a vacuum in the tube. Subsequently, the ground agro- or wood-stalk powder was placed in the tube, and carbon dioxide and nitrogen gases were introduced at temperatures above 500 oC in the tube furnace. Eventually, biochar was obtained after the process.
Steam pyrolysis and spray pyrolysis are the major types of pyrolysis methods. Steam pyrolysis uses steam at 600-700 °C temperatures to heat the powdered agricultural waste materials in the quartz tube furnace, while waste materials are directly sprayed into the pyrolytic furnace at high temperatures to form carbon particles in spray pyrolysis. Steam pyrolysis can preserve the natural porosity of waste materials utilized for NC preparation.
Preparation of NCs from Different Agricultural Wastes
CNTs and mesoporous NCs were produced from sugarcane waste and coconut shells, respectively, using the pyrolysis method. Pineapple waste and date palm were utilized to produce NC with a three-dimensional (3D) continuous network of homogeneous meso/micropore structure and porous NC, respectively, using the hydrothermal method.
CNTs and nanocarbons were synthesized from rice husk and nicotine tabacum stems using the microwave irradiation method and carbonization method, respectively. NC with a honeycomb structure was produced from lapsi seed stone and rubber seed shell using the chemical activation method. NCs with a sheet‐like structure were synthesized from orange peels using the chemical activation together with the pyrolysis method.
![SEM images for nanocarbons carbonized at 400 °C for different time intervals (a) 2 h, (b) 3 h, and (c) 4 h. Adapted with permission from Ref. [105].](https://d12oja0ew7x0i8.cloudfront.net/images/news/ImageForNews_59256_16545066905843106.png)
SEM images for nanocarbons carbonized at 400 °C for different time intervals (a) 2 h, (b) 3 h, and (c) 4 h. Image Credit: Singh, J et al., Materials
Application of NCs Derived from Agro-waste
Wastewater treatment, biosensors, polymer nano-composite materials, and energy storage devices are the major applications of NCs. Nanocarbons produced from agricultural waste have gained considerable prominence owing to their non-toxicity, high chemical inertness, and low cost. NC derived from pineapple leaf using chemical activation agents such as potassium hydroxide is used as supercapacitors or electrochemical capacitors.
Several carbon-based nanomaterials, such as carbon nanofibers, graphene, and CNTs, were synthesized from different secondary agricultural sources. Nanocarbons functionalized with different organic molecules are suitable for detecting adsorbates.
Taken together, agro-waste materials could be used effectively to synthesize NC using different synthesis methods such as HT and simple carbonization, which can reduce the need for natural resources to produce NCs used in different applications and promote sustainability.
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.
Source:
Singh, J., Singh, V., Jirimali, H. et al. Nano‐Structured Carbon: Its Synthesis from Renewable Agricultural Sources and Important Applications. Materials 2022. https://www.mdpi.com/1996-1944/15/11/3969