Regarding glass-forming ability (GFA), Pd42.5Ni7.5Cu30P20 (PNCP) is regarded as the best bulk metallic glass, although the atomic arrangements that result in its attributes are still unknown.
 Researchers compared the atomic configurations of PNCP with other metallic glasses PNP and PCP to find which characteristic atomic configurations of PNCP lend it its excellent glass forming ability. Image Credit: Prof. Shinya Hosokawa from Kumamoto University
Researchers compared the atomic configurations of PNCP with other metallic glasses PNP and PCP to find which characteristic atomic configurations of PNCP lend it its excellent glass forming ability. Image Credit: Prof. Shinya Hosokawa from Kumamoto University
A recent analysis of the atomic configurations of PNCP and comparison with earlier alloys by a global team of researchers under the direction of Prof. Shinya Hosokawa of Kumamoto University, Japan, revealed its distinctive configurations and the source of its GFA. This could aid in the improvement of metallic glasses.
Metallic glass is a ground-breaking substance that blends the strength of metals with the flexibility of glass. These are created by rapidly cooling a liquid metal or alloy so that the atoms do not freeze in the familiar pattern of metals but rather in a random pattern resembling liquids.
Glass also exhibits this erratic liquid-like pattern, which gave the substance its name. Due to its superior glass-forming abilities (GFA), Pd42.5Ni7.5Cu30P20 (PNCP) is regarded as the champion of metallic glasses.
This alloy performs significantly better than both of its parent alloys, Pd40Ni40P20 (PNP), which displays a somewhat worse GFA, and Pd40Cu40P20 (PCP), which displays a significantly worse GFA than PNCP. However, the atomic arrangements that distinguish PNCP and make it a superior metallic glass remained a mystery.
A group of scientists from Japan, Germany, France, Hungary, and the UK recently published a paper that took us one step closer to the answer in Volume 596 of the Journal of Non-Crystalline Solids on November 15th, 2022.
The study was made available online on August 25th, 2022. They successfully examined the atomic structure of PNCP and compared it to the atomic structures of PNP and PCP to uncover the distinctive configurations that could contribute to the outstanding GFA of PNCP.
While previous studies have tried to formulate rules that can predict the GFA of metallic glasses, these have not been experimentally verified. Our findings show that these rules might not be true. Moreover, our research shines a light on the next steps that must be taken to understand how the atomic configurations of other metallic glasses affect their GFA.
Shinya Hosokawa, Professor, Institute of Nanomaterials, Kumamoto University
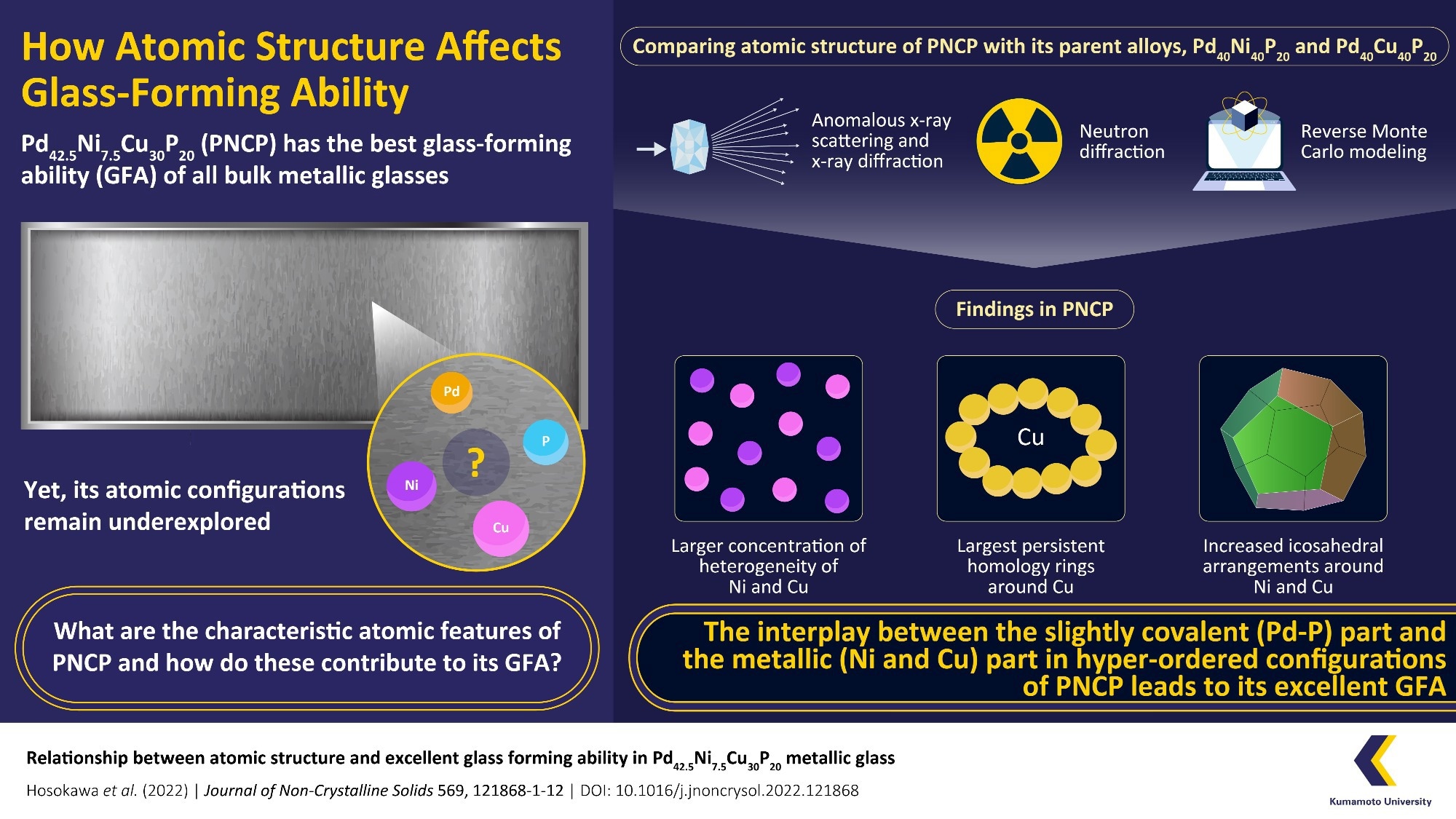
The atomic configurations of a PNCP sample with a 3 mm diameter were elucidated using a variety of observation techniques, including anomalous X-ray scattering, X-ray diffraction, and neutron diffraction. Reverse Monte Carlo modeling techniques were used to analyze these. The observations were then compared to those from PNP and PCP that were similar.
The study discovered definite variations between the atomic configurations of PNCP and its parent alloys. To begin with, as compared to the secondary metals in PNP (nickel) and PCP (copper), PNCP showed more variability in its nickel and copper secondary metals.
In PNCP, copper and nickel were not distributed equally. Additionally, the team noticed that nickel and copper had more icosahedral configurations in PNCP. Last but not least, research using persistent homology (PH), a technique for calculating the topology of compounds, revealed that PNCP contained the largest copper PH rings of all palladium-based bulk metallic glasses.
From these results, the researchers deduced that the superior GFA of PNCP results from the interaction between the metallic component (made of nickel and copper) and the somewhat covalent part (composed of palladium and phosphorus, or Pd-P), which is present in PNCP.
Our results can help others understand the origins of excellent glass-forming ability in many metallic glasses, not just PNCP. Further research can build upon ours and help develop better metallic glasses in the future.
Shinya Hosokawa, Professor, Institute of Nanomaterials, Kumamoto University
Metallic glasses are strong, flexible, and corrosion-resistant. As a result, the utilization of these materials has enormous potential. This type of development can increase the use of metallic glasses in modern society and move a step closer to realizing the promise of these unusual materials.
Funding
- Japan Society for the Promotion of Science (JSPS): Grant-in-Aid for Transformative Research Areas (A) ‘Hyper-Ordered Structures Science’ (Nos. 20H05878 and 21H05569)
- Japan Society for the Promotion of Science (JSPS): Grant-in-Aid for Scientific Research (C) (No. 22K12662)
- Japan Science and Technology Agency (JST) CREST (No. JPMJCR1861)
- Deutsche Forschungsgemeinschaft (DFG) Mercator Fellowship in FOR 2824
- Royal Society–EPSRC Dorothy Hodgkin Research Fellowship
Journal Reference
Hosokawa, S., et al. (2022) Relationship between atomic structure and excellent glass forming ability in Pd42.5Ni7.5Cu30P20 metallic glass. Journal of Non-Crystalline Solids. doi:10.1016/j.jnoncrysol.2022.121868.