Hydrogen is considered a future energy source—at least when it is created in a climate-friendly way. Also, hydrogen can be essential for the creation of active ingredients and other essential substances. To produce hydrogen, water (H2O) can be transformed into hydrogen gas (H2) through a sequence of chemical processes.
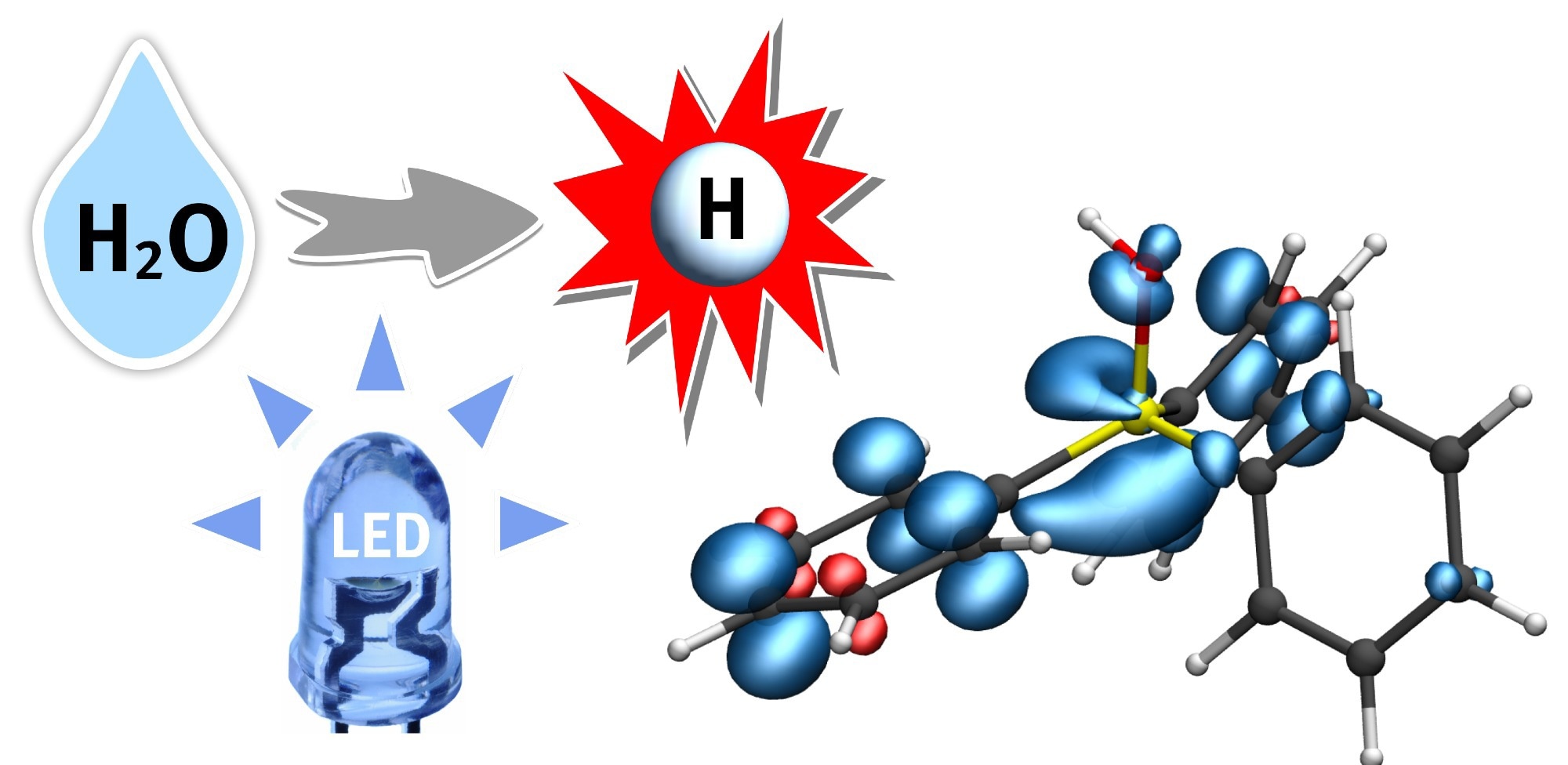
A hydrogen atom (H) from water (H2O) is transferred to a phosphine-water radical cation under the supply of light energy (LED). This important radical intermediate can further transfer the hydrogen atom (white) to the substrate. The blue regions indicate the electron spin distribution. Image Credit: University of Munster
Nevertheless, because water molecules are very stable, splitting them into oxygen and hydrogen seems to be a huge difficulty for chemists. For it to be efficient at all, firstly, the water needs to be activated with the use of a catalyst—then it reacts more effortlessly.
A study group headed by Prof. Armido Studer at the Institute of Organic Chemistry at Münster University has formulated a photocatalytic process where water, under mild reaction conditions, is activated via triaryl phosphines and not, like in a majority of other processes, via transition metal complexes.
Now, this strategy has been published in the “Nature” journal. It will pave a new path to the active field of research associated with radical chemistry, states the team. Generally, radicals are highly reactive intermediates. The team employs a special intermediate—a phosphine-water radical cation—as activated water, from which hydrogen atoms from H2O can be seamlessly divided off and moved to a further substrate. Light energy drives the reaction.
Our system offers an ideal platform for investigating unresearched chemical processes which use the hydrogen atom as a reagent in synthesis.
Prof. Armido Studer, Institute of Organic Chemistry, Münster University
Dr. Christian Mück-Lichtenfeld, who examined the activated water complexes with the use of hypothetical methods, states, “The hydrogen-oxygen bond in this intermediate is extraordinarily weak, making it possible to transfer a hydrogen atom to various compounds.” Dr. Jingjing Zhang, who performed the experimental work, states: “The hydrogen atoms of the activated water can be transferred to alkenes and arenes under very mild conditions, in so-called hydrogenation reactions.”
Hydrogenation reactions are extremely essential in the agrochemical industry, in pharmaceutical research, and in materials sciences.
The work was funded by Alexander von Humboldt Foundation.
Journal Reference
Jingjing Zhang, et al. (2023): Photocatalytic phosphine-mediated water activation for radical hydrogenation. Nature. doi.org/10.1038/s41586-023-06141-1.