Dec 17 2009
A new carbon support that greatly increases the durability of proton-exchange membrane fuel cells has been developed by scientists at Pacific Northwest National Laboratory and Princeton University. This new material significantly improves the stability of the fuel cell catalyst and will potentially lower the cost of these fuel cells. This breakthrough research hit number one on the most-downloaded list of Electrochemistry Communications articles this fall.
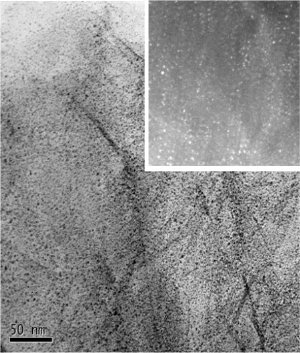 Transmission electron microscopic image of small platinum catalyst particles supported on a functionalized graphene sheet.
Transmission electron microscopic image of small platinum catalyst particles supported on a functionalized graphene sheet.
Currently, proton-exchange membrane fuel cells are not widely used because of the high manufacturing cost and relatively low endurance. To be commercially viable, the cost needs to be dramatically reduced. These new carbon supports might do just that.
Inside today's fuel cell, platinum catalyzes the reaction. The conditions inside the fuel cell are pretty harsh: high pressure, high temperature. Under these conditions, some of the platinum particles fly off the support, making them unavailable to speed the reactions. Some of the particles clump together. When this happens, the particles present less surface area. And, it is on the surface where the reaction happens. So, less surface area, less catalysis. The team investigated a new type of support.
For this study, they sliced graphite, similar to the carbon in a pencil, into single atomic layers to form dense wrinkled sheets called functionalized graphene sheets.
Then, they treated these sheets with the platinum catalyst. Using a transmission electron microscope, they saw the difference in how the catalysts particles were attached to the graphene sheets and a commercial support. The images clearly showed a uniform distribution of much smaller platinum nanoparticles on the graphene. Using an X-ray photoelectron spectrometer, they proved the graphene has more functional groups available to bind the platinum catalyst compared to the commercial support. Both of these instruments are at DOE's EMSL, a scientific user facility located at PNNL.
Their conclusion was that the graphene sheets have a stronger metal-support interaction and produced smaller catalysts particles that were more resistant to degradation. Functionalized graphene sheets could potentially lead to a more stable, efficient, and lower-cost fuel cell.
This study lays the foundation for future work with this promising carbon material. Future research will focus on increasing the efficiency of the material fabrication and the durability of the graphene sheets.
More information: R Kou, Y Shao, D Wang, MH Engelhard, JH Kwak, J Wang, VV Viswanathan, C Wang, Y Lin, Y Wang, IA Aksay, and J Liu. 2009. "Enhanced activity and stability of Pt catalysts on functionalized graphene sheets for electrocatalytic oxygen reduction." Electrochemistry Communications, Volume 11, Issue 5, May 2009, Pages 954-957.