Feb 15 2016
The prevailing chilly season is known to change Nebraska into an outdoor ice skating rink. During this cold environment, a University of Nebraska (UNL)-Lincoln-led research group have proposed a new form of the slippery water form, which even Mother Nature was not aware of.
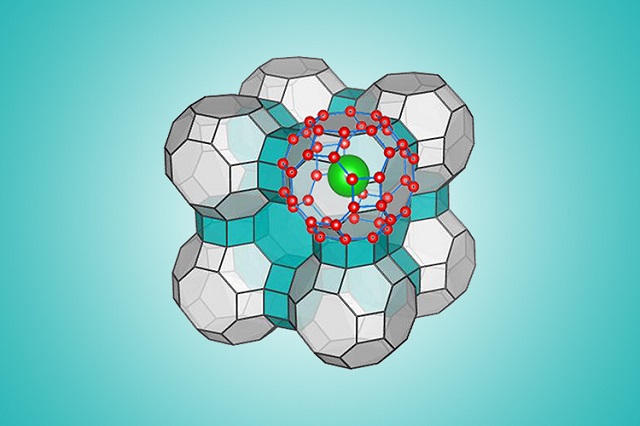 This illustration shows the ice's molecular configuration. (Courtesy photo/Yingying Huang and Chongqin Zhu).
This illustration shows the ice's molecular configuration. (Courtesy photo/Yingying Huang and Chongqin Zhu).
The new form of ice, reported in the Feb. 12 issue of Science Advances, has about 25% lower density than the low form of ice produced by a European team in 2014.
If the new ice is able to be synthesized, then it would be the 18th known water crystal – and the first type to be discovered in the United States, since before World War II.
We performed a lot of calculations (focused on) whether this is not just a low-density ice, but perhaps the lowest-density ice to date. A lot of people are interested in predicting a new ice structure beyond the state of the art.
Xiao Cheng Zeng, Professor of Chemistry, Ameritas University
This discovery symbolizes the latest in ice-related research work from Zeng. Earlier, Zeng had discovered a two-dimensional "Nebraska Ice," which contracts, instead of expanding, if frozen under specific conditions.
Zeng's latest work, which was co-led by Dalian University of Technology's Jijun Zhao, utilized molecular simulation and a computational algorithm to determine extreme temperature and pressure ranges, where water can freeze in a predicted arrangement. The predicted configuration is a clathrate – which contains water molecules formed as an interlocking cage-like structure.
An earlier thought was that these cage-like structures preserve their structural form only when they have “guest molecules” amongst them, such as those of methane. Natural clathrates existing in permafrost and on the ocean floor, contain methane. Like the earlier European research, Zeng and his team worked out that their clathrate retains its stability even after the removal of guest molecules.
More efforts are needed before the clathrate can be synthesized. According to the team’s calculations the new type of ice can be formed when molecules of water are kept in an enclosed space, and very high outwardly expanding pressure is applied to the space.
The amount of expansion pressure needed by the space for this purpose at -10°F would be more than four times of the pressure existing in Pacific Ocean’s deepest trench. At -460°F the pressure would be approximately equal to an individual, at sea level, carrying 300 jumbo jets.
The guest molecules have to be extracted through a vacuuming method, which was developed by the European team. Zeng and his team were inspired by this extraction method to conduct their new research work.
Another motivation factor for Zeng’s team was the wonders of ice – the form covering this planet for billions of years.
Water and ice are forever interesting because they have such relevance to human beings and life. If you think about it, the low density of natural ice protects the water below it; if it were denser, water would freeze from the bottom up, and no living species could survive. So Mother Nature's combination is just so perfect.
Xiao Cheng Zeng, Professor of Chemistry, Ameritas University
Upon confirmation, the new form of ice would be named as “Ice XVII” – a naming quirk resulting from researchers calling the first two identified forms as “Ice I.”
Zeng and Zhao co-authored the Science Advances study with UNL postdoctoral researcher Chongqin Zhu; Yingying Huang, a visiting research fellow from the Dalian University of Technology; and researchers from the Chinese Academy of Sciences and the University of Science and Technology of China.
The National Science Foundation partially funded the research, and UNL's Holland Computing Center provided assistance to the research.