The latest research in the journal Coatings is focused on new ways of improving the superhydrophobic characteristics of zinc oxide (ZnO) and titanium dioxide coatings (TiO2).

Study: New Approaches to Increasing the Superhydrophobicity of Coatings Based on ZnO and TiO2. Image Credit: Yasni/Shutterstock.com
According to Muslimov and his team, synthetic coatings with a non-polar lattice structure, high melting point, and strong adherence to ZnO, as opposed to commonly utilized coating materials with poor performance attributes, are being employed in new ways.
Industrial Utilization of Zinc and Titanium Oxide
Zinc and titanium oxide-based technologies are employed in a variety of sectors, ranging from current electronics and photonics equipment that function under a variety of circumstances (low and high temperatures and pressures, different gases, etc.) to bioanalytical gadgets and biosensors. Special consideration is given to the use of zinc and titanium oxides in hydrogen power distribution systems.
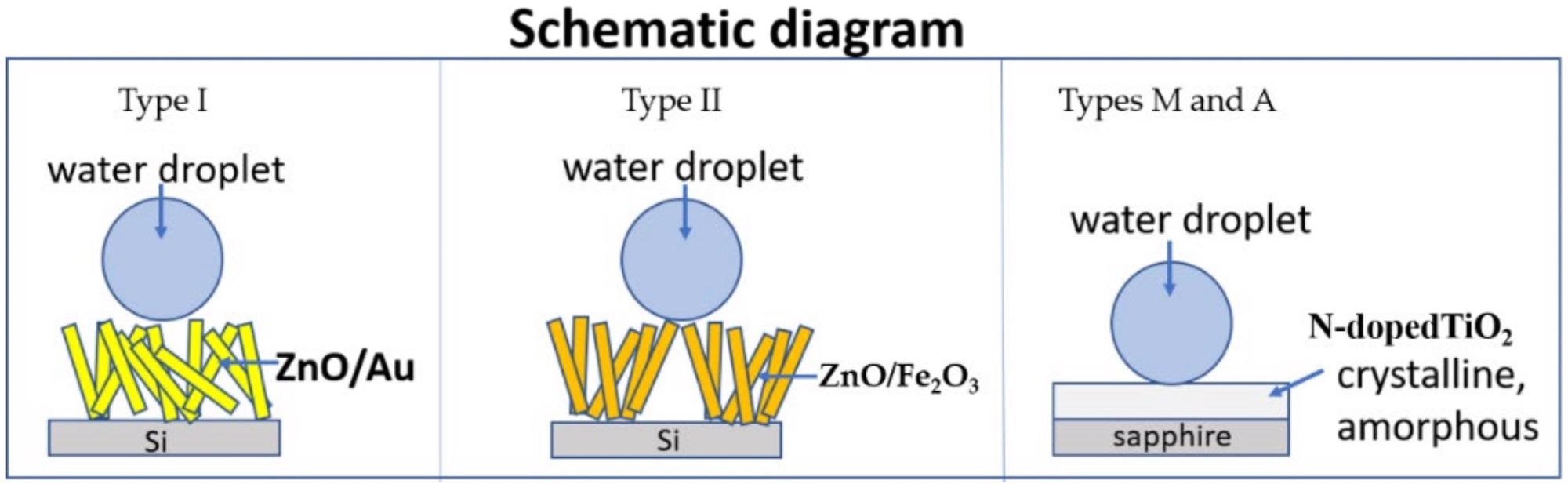
Schematic diagram. Image Credit: Muslimov, A. E et al., Coatings
Properties of Materials
The surface of zinc oxide (wurtzite crystal structure) is very hydrophilic (wetting contact angle less than 5), and even lengthy processing at 350 C does not result in detectable alterations. This is owing to the properties of water molecules interacting with a ZnO surface. ZnO is characterized by polarization and, as a result, enhanced surface potential due to crystal structural peculiarities.
TiO2 has heterogeneity and may dwell in three states: anatase, brookite, and rutile. Rutile is the elevated stable structure. By precisely directing the reaction conditions, TiO2 coatings with required traits may be created. Titanium is less flammable than zinc, thus it is capable of achieving a pure titanium coating first and then treating it in plasma in an open environment.
To improve a solid surface's hydrophobicity, the free surface energy at the three-phase contact point must be reduced, according to the classical Youngs’ equation.
Experiment Methods
Catalytic fabrication from the vapor form produced ZnO samples with bimodal ruggedness (sample type I) of the surface on sapphire substrates. A characteristic of the synthesizing was a rapid drop in temperature in the region of zinc evaporation 17 minutes after the production began.
Circumstances for the production of type II ZnO samples: a structured Au lattice was provisionally constructed in the form of square cells with a period of 8 m, on which a ZnO microstructure was generated under the aforesaid parameters (in the mode of the type I sample).
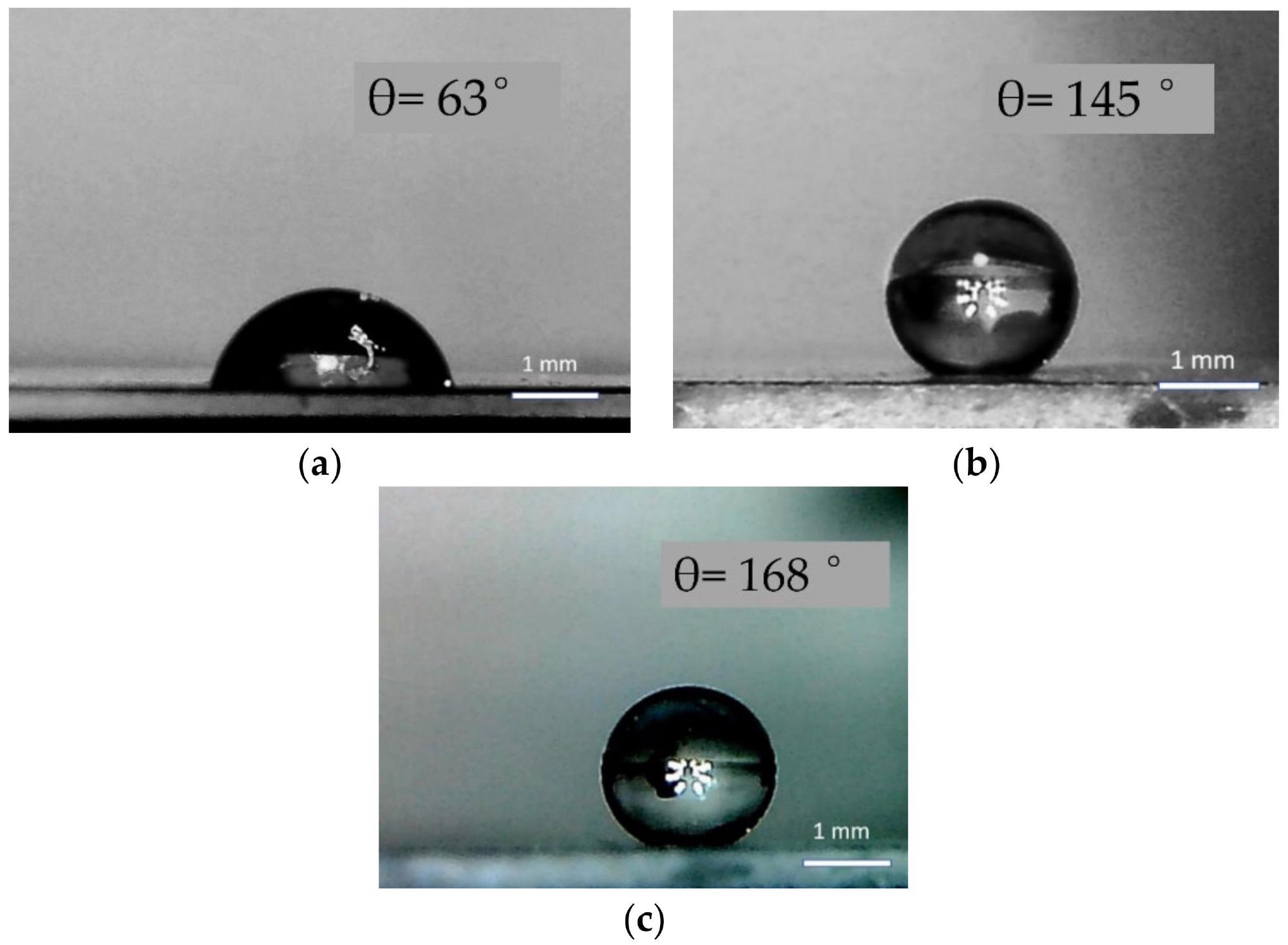
The shape of water droplets and contact angles on the surface of a pure Au film (a), ZnO samples of type I pure (b), and with Au (c). Image Credit: Muslimov, A. E et al., Coatings
To make TiO2 specimens, a titanium layer (500 nm thick) was first created on a sapphire substrate using magnetron sputtering, then the specimen was processed in an open environment with a flow of low-temperature high-enthalpy nitrogen plasma.
Research Findings
According to scanning electron measurements, the type I specimen was an aggregation of hexagonal ZnO microrods up to 1 m in diameter, from the extremities of which sharpened ZnO nanorods arched. The nanorods measured 3–4 m in length and 100–150 nm in diameter.
The diameter of the developing ZnO structures is regulated under low oxygen circumstances by regulating the conditions in the zinc evaporation zone. At the last step of the production, the diameter of the ZnO rods is reduced by reducing the temperature in the zone of the zinc evaporator.
The hydrophobic characteristics of the generated specimens' surfaces were studied. The wetting contact area for the sample material of Au film on sapphire was 63, which fits with the findings. In the region of covering of a ZnO framework with a gold layer, superhydrophobicity (a rise in the contact angle to 168) is found. The narrow sliding inclination of the drop is a key feature describing the superhydrophobic condition.
The non-polar M-plane forms the lateral surfaces of the ZnO hexagonal rods. Water particles collect and disperse on the M-planes of ZnO, resulting in an interface that is hydrophilic and durable.
A Type II ZnO sample's pristine exterior has hydrophobic characteristics. Because of the synthesis characteristics, a ZnO surface with bidirectional ruggedness is created, allowing the "lotus effect" to be realized. After covering a ZnO type II specimen with a layer of Fe2O3, the contact angle achieved a new high of 173.
Limitations
Although the study discusses advantageous features, a major problem exists. It is difficult to increase the hydrophobicity of the interface of ZnO-based products. When utilized to promote hydrophobicity, polymer coverings with strong adherence stand out due to their technological intricacy, significant financial impact, and fairly low dissolution temperatures.
The limits resulting from the use of these methods include sustainability and coating homogeneity, both of which are solely practical issues.
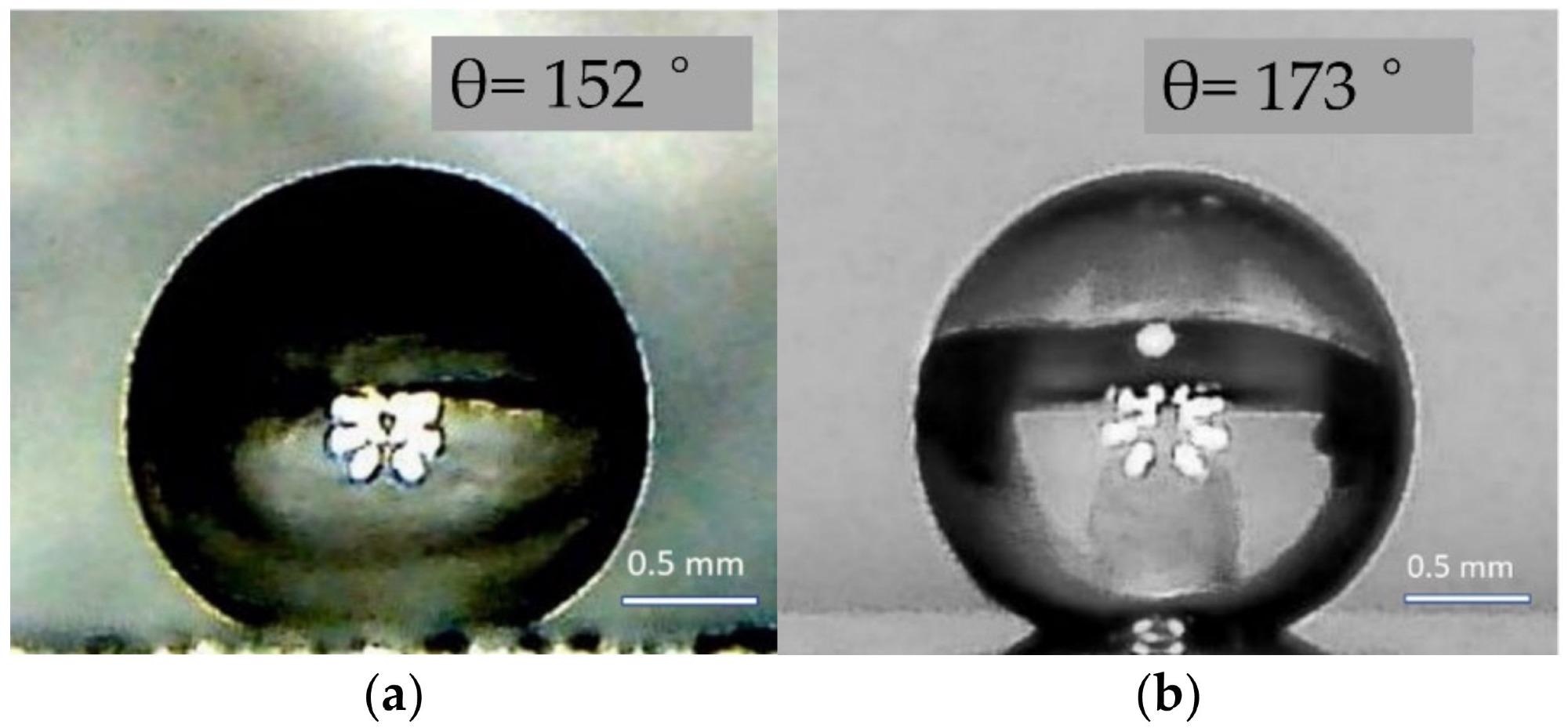
The shape of water droplets and the magnitude of contact angles on the surfaces of pure type II ZnO (a) and with a coating of Fe2O3 (b). Image Credit: Muslimov, A. E et al., Coatings
In short, the study has proved that an improvement in hydrophobicity can result in an improvement in photocatalysis performance as well as device endurance. Technological regulation, however, is essential for TiO2 substances. In short, the superhydrophobicity of ZnO and TiO2 has been thoroughly discussed in detail.
References
Muslimov, A. E., Gadzhiev, M. K., & Kanevsky, V. M. (2021). New Approaches to Increasing the Superhydrophobicity of Coatings Based on ZnO and TiO2. Coatings MDPI. https://www.mdpi.com/2079-6412/11/11/1369
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.