 By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Apr 20 2022
By Surbhi JainReviewed by Susha Cheriyedath, M.Sc.Apr 20 2022In a review recently published in the open-access journal Polymers, researchers discussed the sustainable and potential applications of animal waste proteins.

Study: Sustainable Applications of Animal Waste Proteins. Image Credit: Ingrid Pakats/Shutterstock.com
Background
Agriculture is one of the most important economic sectors growing and developing year after year in response to human needs. Animal by-products are high in nitrogen-containing chemical substances, such as keratin and collagen, which are fibrillar proteins. Keratin and collagen can be used in biomedicine and biotechnology owing to their unique features.
Organic waste is being disposed of in a variety of ways. Incineration and landfilling are both frequent low-resource and low-energy processes. However, such methods spread diseases, pollute the environment, and hinder the utilization of important oligopeptides, proteins, and amino acids from agricultural by-products.
As a result, there is a requirement for the discovery and development of novel sustainable methods for the recycling of animal waste. Carbonization is an energy-intensive yet promising technology for processing agricultural leftovers.
The study of microorganisms that synthesize keratinolytic and collagenolytic proteases promotes new promising advancements in methods for the processing and isolation of protein polymers from aquaculture waste and animal farming that align with the trends of a sustainable economy, to mitigate the burden on the environment by using biodegradable non-hazardous agents, and to increase the target product yield accuracy because of the specificity.
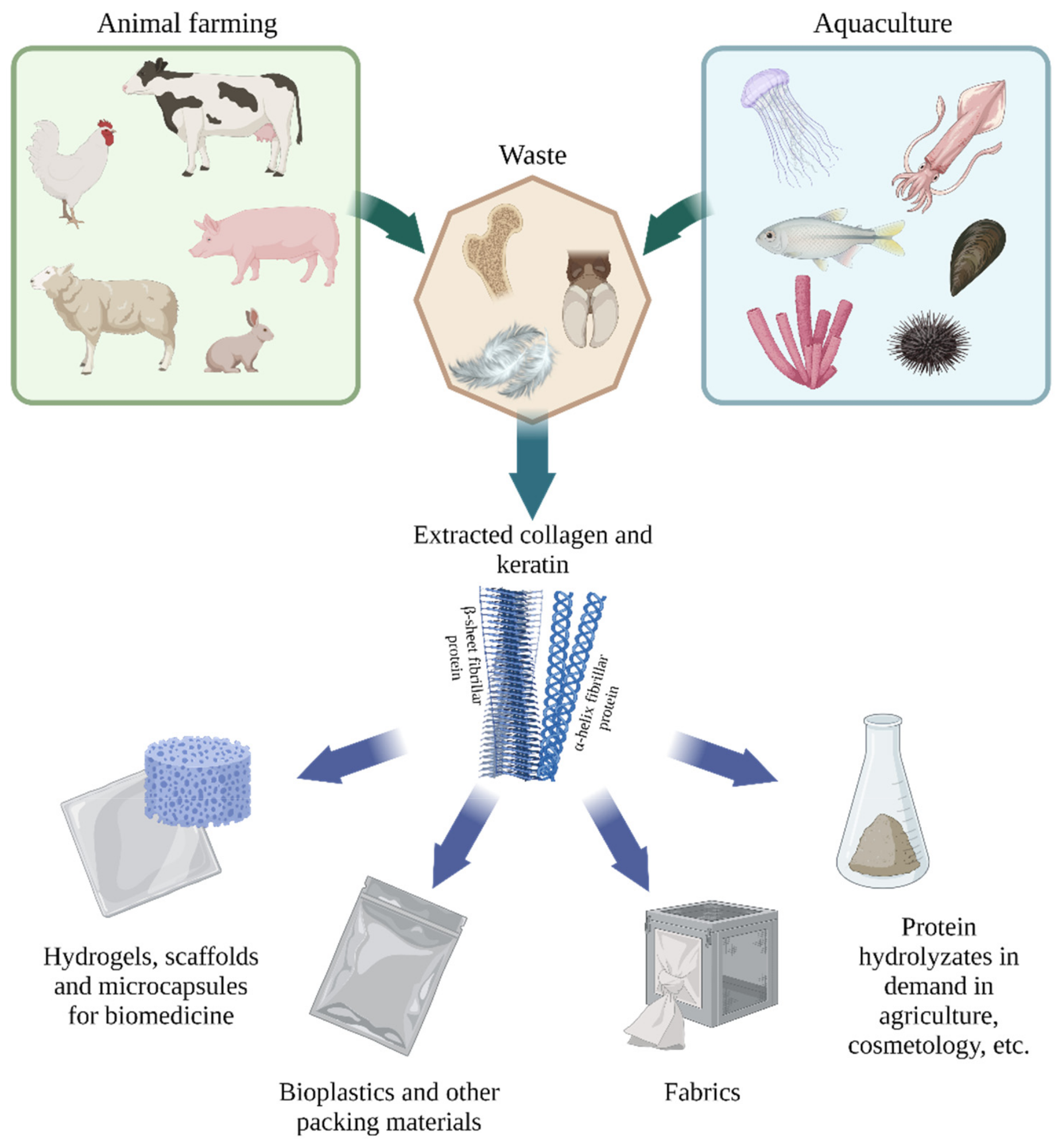
Sources and applications of collagen and keratin (created with BioRender.com, access date: 9 November 2021). Image Credit: Timorshina, S et al., Polymers
About the Study
In this study, the authors discussed the impact of increased access to and consumption of food products on the number of by-products produced and on the development of processing technologies. The biotechnological potential of collagen and keratin that make up a large portion of the animal origin protein waste stream was also discussed.
The demand for collagen and keratin in bioengineering in various forms and formats, such as nanoparticles, hydrogels, and scaffolds for targeted drug delivery and regenerative medicine, films for the development of biodegradable packaging materials, and so on, due to their unique fibrillar structure, was illustrated.
The researchers discussed several sustainable sources of collagen and keratin, as well as the multiformity of these proteins' applications. The current data on the biochemical performance of fibrillar proteins derived from agricultural by-products— keratin and collagen—as well as the possibilities of using such substances as the secondary raw materials in a variety of fields, such as implant manufacturing, degradable packaging materials, and bioremediation components, were demonstrated. The importance of waste proteins from animal agriculture and aquaculture for the development of many sectors of the economy was demonstrated.
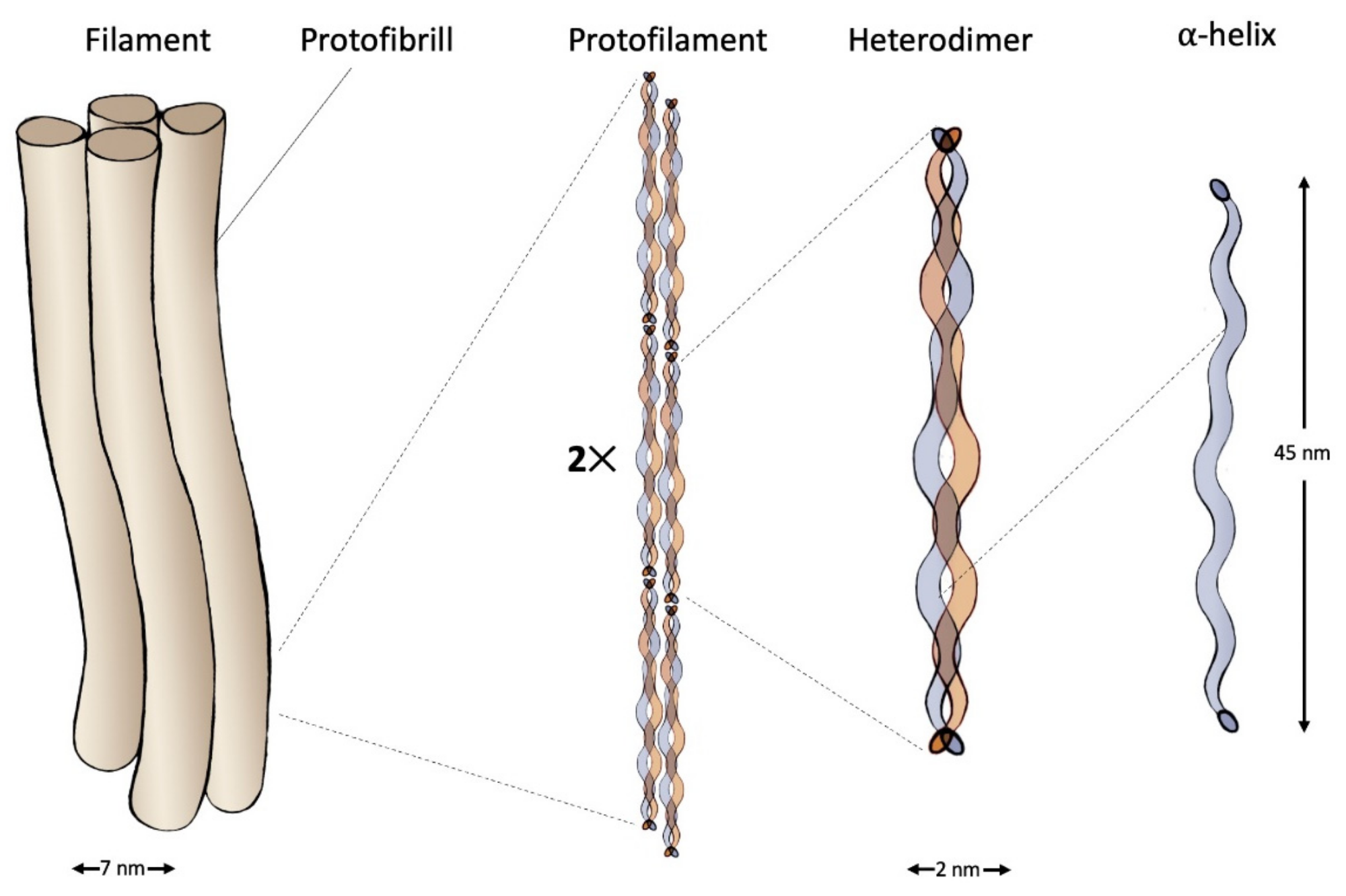
Structure of an intermediate filament composed of IF-keratin. Image Credit: Timorshina, S et al., Polymers
Observations
Several studies demonstrated that the peptides produced by keratin hydrolysis inhibit the growth of microorganisms, including phytopathogens. Collagen hydrolysate was found to be a combination of short peptides with low molecular weight in the range of 3–6 KDa produced by further enzymatic hydrolysis.
The COL29A1 gene was identical to the COL6A5 gene, and the α1(XXIX) chain corresponded to the 5(VI) chain for epidermal collagen XXIX. Type I collagen was found to be the most well-studied at the time, as well as the predominant fibrillar protein of connective tissue. Type I collagen accounted for over 70% of the total amount of collagen in the body, making it the most frequent collagen employed in the manufacture of various biomaterials.
Proline (28%) and 4-hydroxyproline (38%) were the most common amino acids in the Xaa and Yaa locations, respectively. In collagen, ProHypGly was the most abundant triplet (10.5%). CBPs ranged in size from 8 to 25 kDa and, like IF-keratins, had three primary domains: central, C-, and N-terminal. One of the studies reported that due to a rotation of around 45°, beta-sheet dimers formed a helical shape filament. Blood rich in fibrinogen accounted for 4–7.5% of the total weight of poultry and livestock, and protein content in the blood varied by animal species but seldom surpassed 30%.
Collagen was reported to be the most abundant protein in mammals, which accounted for more than 30% of the total protein content of the body. Keratin made up to 90% of the entire weight of skin derivatives like wool and feathers, which made its extraction easier.
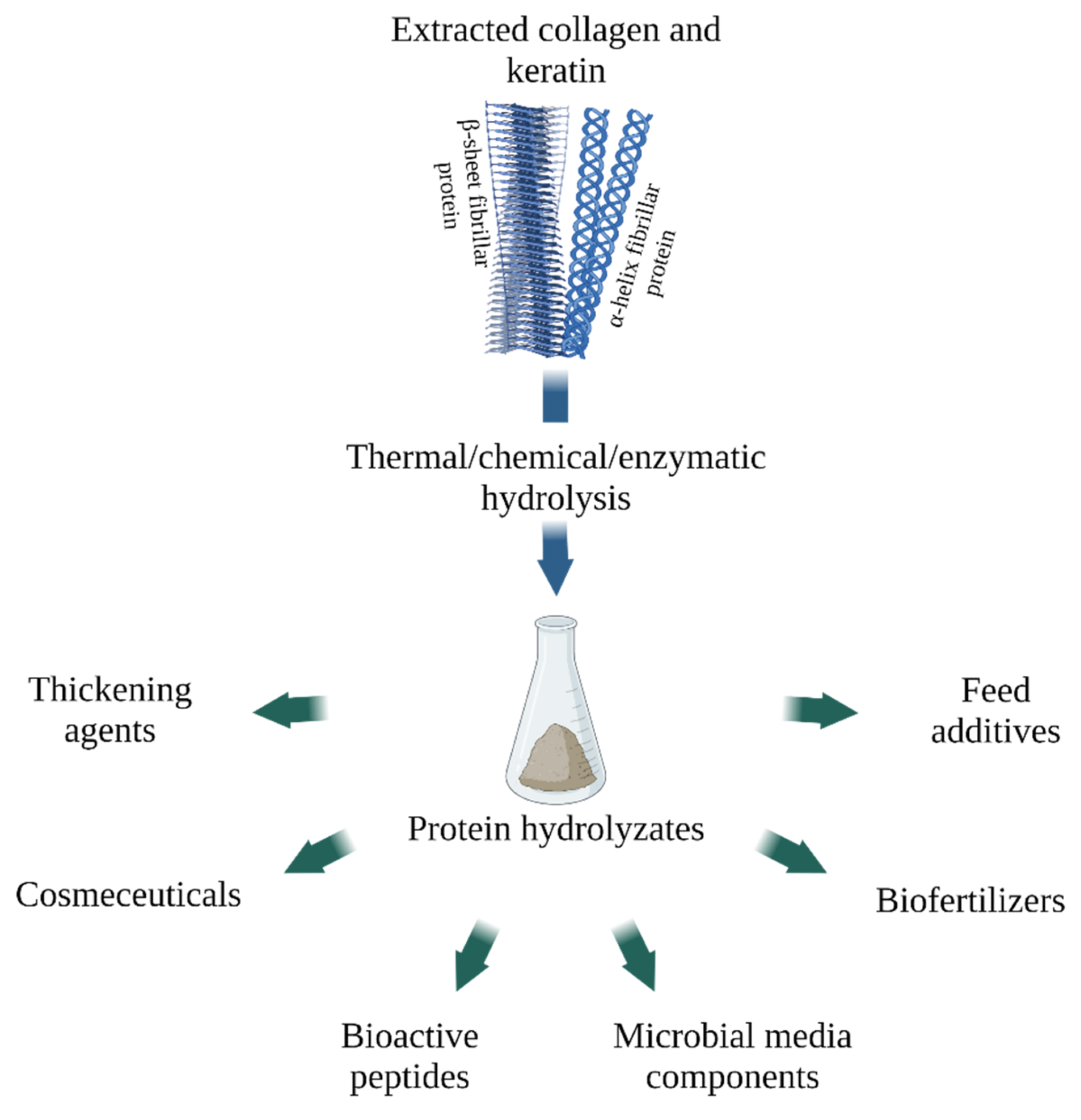
Applications of collagen and keratin hydrolysates (created with BioRender.com, access date: 9 November 2021). Image Credit: Timorshina, S et al., Polymers
Conclusions
In conclusion, this study elucidated that collagen and keratin are low-cost material synthesis substrates with qualities that could fulfill the needs of modern agriculture, food, biomedicine, and textile sectors. Recycling fibrillar proteins derived from by-products lowered the cost of the product as well as the environmental impact by reducing waste and facilitating a transition toward rational, sustainable resource usage.
The authors emphasized that the advancement of techniques for extracting and processing collagen and keratin from organic waste will allow biodegradable composites with specified qualities to be created, which will fulfil the green economy trend and open up new applications.
Source
Timorshina, S., Popova, E., Osmolovskiy, A., Sustainable Applications of Animal Waste Proteins. Polymers 14(8) 1601 (2022). https://www.mdpi.com/2073-4360/14/8/1601
Disclaimer: The views expressed here are those of the author expressed in their private capacity and do not necessarily represent the views of AZoM.com Limited T/A AZoNetwork the owner and operator of this website. This disclaimer forms part of the Terms and conditions of use of this website.