Reviewed by Alex SmithAug 1 2022
For applications like the power grid, aqueous zinc batteries have the capacity to play an important role in future energy storage systems due to their economical cost and substantiality. However, the advancement of this technology has been put on hold as a result of a safety concern.
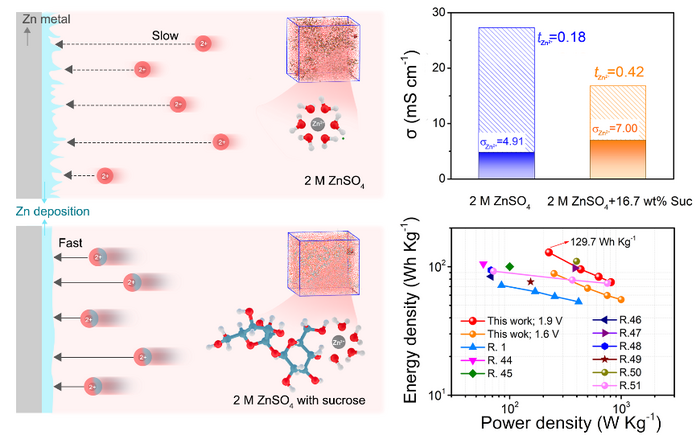 Researchers designed a sucrose-modified aqueous electrolyte that increases the mobility of zinc ion in response to the electric field and successfully achieves dendrite-free zinc batteries without compromising electrochemical performance. Image Credit: Nano Research, Tsinghua University.
Researchers designed a sucrose-modified aqueous electrolyte that increases the mobility of zinc ion in response to the electric field and successfully achieves dendrite-free zinc batteries without compromising electrochemical performance. Image Credit: Nano Research, Tsinghua University.
To stabilize secure future applications and the zinc ion environment, Chinese scientists established a solution, incorporating chemically modified table sugar. This study was published in Nano Research, on July 28th, 2022.
An increasingly diverse range of power-hungry applications continues to boost demands for large-scale, low-cost energy storage — ranging from electric cars to wind and solar power systems. According to the research, aqueous zinc (Zn) batteries have become the top choice as one of the more hopeful options for meeting the demand sustainably.
They are high safety and cost-effective compared to current lithium-ion batteries with flammable organic electrolytes. In addition, Zn anode presents super high theoretical capacity, which makes these Zn batteries even more promising for applications like future grid energy storage.
Meinan Liu, Study Author and Associate Professor, Nano-tech and Nano-bionics, University of Science and Technology of China
Nonetheless, when the zinc ion (Zn2+) concentration on the surface of the anode falls to zero, dendrites start developing. Electrochemical performance deteriorates as a result of uncontrolled Zn dendrite growth and becomes a risk to safe operation.
“These dendrites can penetrate the separator and cause the battery to short-circuit,” Liu stated.
Previous studies have focused on adjusting the solvent environment, which is called “solvation structure.” It can enhance the mobility of Zn2+ as a result of the electric field effectively suppressing the dendrites’ growth. The challenge was that these past adjustments, such as including fewer water molecules or introducing other salts, have also ended up reducing the system’s ionic conductivity.
Between Zn2+ solvation structure and its mobility, there was a basic gap in the researcher's understanding; one important characteristic seemed to affect the stability of the Zn anode and the growth of dendrite.
A collective study team from several Chinese institutions attempted a new tactic as an effort to bridge this gap. To adjust the solvation structure of Zn2+, the team introduced common table sugar with multiple hydroxyl groups (a hydrogen and an oxygen bound together) into the electrolyte.
It confirmed that the sucrose molecules improved movement and curbed dendrite growth without compromising on stability by performing atomistic simulations and experiments. This method, in fact, offered unanticipated advantages as well:
Findings confirm that sucrose molecules in the solvation sheath not only enhance the mobility, ensuring fast Zn2+ kinetics, but also protects the Zn anode from water corrosion and successfully achieves Zn dendrite-free deposition and side reaction suppression.
Meinan Liu, Study Author and Associate Professor, Nano-tech and Nano-bionics, University of Science and Technology of China
This establishes the promising capacity of employing this simple sucrose-modification for high-performance zinc batteries in the future and takes the research field a step closer to the researchers ultimate goal of attaining a safe, green, high-performance Zn battery.
“Hopefully this safe, low-cost Zn battery could be applied in grid energy storage,” Liu stated.
This method also offers itself to added changes and alterations: Zn-carbon cells provide higher energy density and enhanced stability, which suggest a promising possible application of sucrose-altered electrolytes for Zn batteries in future.
Possible roadblocks and use cases for aqueous zinc batteries, especially how they might manage extreme temperatures, will be considered by the researchers in future studies.
“The aqueous electrolyte of Zn battery will be frozen in low temperature, so we are looking into how to address the temperature influence on battery performance,” Liu concluded.
Other colloborators are Yufang Cao, Linge Li, Haifeng Tu, Yuzhen Hu, Shuang Cheng, Hongzhen Lin, Jiangtao Di, and Yongyi Zhang from the School of Nano-Tech and Nano-Bionics, University of Science and Technology of China; Yufang Cao, Xiaohui Tang, Linge Li, Haifeng Tu, Yuzhen Hu, Shuang Cheng, Hongzhen Lin, Jiangtao Ii, and Yongyi Zhang are also associated with the Key Laboratory of Multifunctional Nanomaterials and Smart Systems, Advanced Materials Division, Suzhou Institute of Nano-Tech and Nano-Bionics at the Chinese Academy of Sciences, as are Xiaohui Tang, Yingying Yu, and Liwen Zhang; Yufang Cao, Liwen Zhang, Jiangtao Di, and Yongyi Zhang are also with the Division of Nanomaterials and Jiangxi Key Lab of Carbonene Materials, Jiangxi Institute of Nanotechnology.
This study was funded by the National Natural Science Foundation of China, National Key Research and Development Program of China, the Science and Technology Project of Jiangxi Province, Outstanding Youth Fund of Jiangxi Province, Jiangxi Double Thousand Talent Program, and Science Technology Major Project of Nanchang.
Journal Reference:
Cao, Y., et al. (2022). Fast Zn2+ mobility enabled by sucrose modified Zn2+ solvation structure for dendrite-free aqueous zinc battery. Nano Research. doi.org/10.1007/s12274-022-4726-3.