A team of researchers conducted a rigorous examination of thermal safety in lithium–sulfur pouch cells. The researchers concentrated their research on the exothermic events that cause the temperature of lithium–sulfur batteries to rise after numerous charge–discharge cycles.
The violent exothermic reactions between high-order polysulfide rather than lower-order polysulfide and dendritic Li push the cycled Li–S pouch cells into thermal runaway. Image Credit: Fengni Jiang, Taiyuan University of Technology
Their research advances the understanding of the thermal behaviors of lithium–sulfur batteries and points the way toward safer, more practical applications.
On December 8, 2022, the researchers published their study in the journal Particuology.
Lithium metal batteries, particularly lithium–sulfur batteries, have received considerable interest because of their high theoretical specific energy density, low cost, and environmental friendliness. These batteries have the potential to be used in electric cars and high-end portable gadgets due to their high energy density.
However, several hurdles must be overcome before lithium–sulfur batteries may be used in practical applications. The “shuttle effect” of intermediate lithium polysulfides between anodes and cathodes, the intrinsic insulating property of sulfur, and dendritic concerns with lithium anodes over cycles are among the challenges.
More importantly, the thermal safety concern caused by the highly active lithium metal is a critical bottleneck for practical uses of lithium–sulfur batteries.
Therefore, it is of great importance to investigate the key exothermic reactions which lead to the dramatic and abnormal temperature rise of lithium-sulfur batteries after several charge-discharge cycles.
Xin-Bing Cheng, Professor, Southeast University
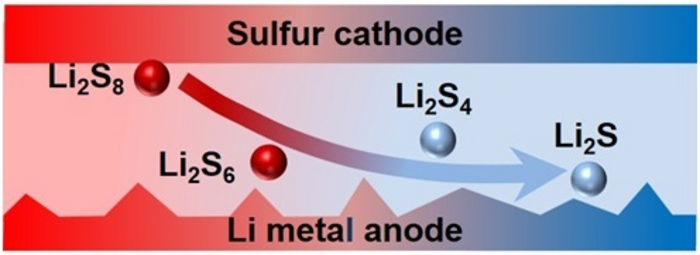
As a result, the investigators decided to look into the thermal safety of thoroughly cycled lithium–sulfur pouch cells. They investigated the thermal runaway mechanisms of lithium–sulfur batteries in depth. Thermal runaway is the process through which a battery overheats. They discovered that thermal runaway in lithium–sulfur pouch cells were caused by interactions between dissolved higher-order polysulfides and lithium metal.
During the accelerating rate calorimeter test, a 16-cycle pouch cell showed great safety, heating from 30 to 300 °C without a significant temperature increase. In comparison, the scientists discovered that heating a 16-cycle pouch cell with added electrolyte to 147.9 °C causes strong exothermic reactions and the generation of enormous heat.
The differing thermal behaviors of these batteries are linked to the variable viscosity of the electrolytes. The high viscosity of the electrolyte leads to poor solvent transport at the electrode-electrolyte interface. This poor solvent diffusion helps to increase the thermal stability of battery components at high temperatures.
The thermal behavior of 45-cycle pouch cells with additional electrolytes, on the other hand, is consistent with that of cells without additional electrolytes.
The team discovered that during the accelerating rate calorimeter test, the highest temperature rate for the lithium–sulfur pouch cell after 45 cycles is only 1.72 °C min−1 and its highest temperature is only 304.0 °C, whereas the numbers for the 16-cycle lithium–sulfur cells with an additional electrolyte are 162.4 °C s−1 and 436 °C, respectively. The diverse polysulfide species in cycled electrolytes are responsible for the varying thermal characteristics of batteries.
We discovered that the strong exothermic reactions between dendritic lithium and dissolved higher-order polysulfide rather than lower-order polysulfide drive the dramatic and abnormal temperature rise of deeply cycled lithium-sulfur pouch cells. Therefore, from a safety viewpoint, it is essential to inhibit the polysulfide shuttle for lithium-sulfur batteries.
Xin-Bing Cheng, Professor, Southeast University
The team’s research methodically uncovered the thermal runaway mechanisms of cycled lithium–sulfur pouch cells.
It is important to develop effective strategies to inhibit the undesirable polysulfide shuttle and dendrite growth in Li-S batteries. Once the polysulfide shuttle and dendrite growth in lithium–sulfur batteries is suppressed completely, the thermal safety of lithium-sulfur batteries can be greatly improved.
Xin-Bing Cheng, Professor, Southeast University
The study group includes Feng-Ni Jiang (Taiyuan University of Technology and Tsinghua University); Shi-Jie Yang, Zi-Xian Chen, Hong Yuan and Jia-Qi Huang (Beijing Institute of Technology); He Liu (Beijing Institute of Technology and Nanjing University of Information Science and Technology); Lei Liu (Taiyuan University of Technology); Xin-Bing Cheng (Southeast University); and Qiang Zhang (Tsinghua University).
The study was financially supported by the National Key Research and Development Program, the National Natural Science Foundation of China, the Beijing Municipal Natural Science Foundation, the Natural Science Foundation of Jiangsu Province, and the Fundamental Research Funds for the Central Universities.